B cells extract and present immobilized antigen: implications for affinity discrimination
- PMID: 10675320
- PMCID: PMC305589
- DOI: 10.1093/emboj/19.4.513
B cells extract and present immobilized antigen: implications for affinity discrimination
Abstract
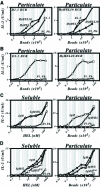
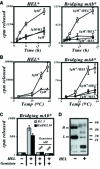
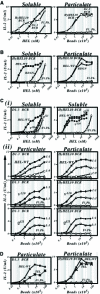
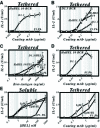
Binding of antigen to B-cell antigen receptor (BCR) leads to antigen internalization and presentation to T cells, a critical process in the initiation of the humoral immune response. However, antigen internalization has been demonstrated for soluble antigen, in vivo antigen is often encountered in insoluble form or tethered to a cell surface. Here, we show that not only can B cells internalize and present large particulate antigen (requiring a signalling-competent BCR to drive antigen uptake), but they can also extract antigen that is tethered tightly to a non-internalizable surface. The form in which the antigen is displayed affects the B cell's ability to discriminate antigen-BCR affinity. Thus, arraying an antigen on a particle or surface allows efficient presentation of low affinity antigens. However, the presentation efficiency of antigen arrayed on an internalizable particle plateaus at low affinity values. In contrast, extraction and presentation of antigen from a non-internalizable surface depends on antigen-BCR affinity over a wide affinity range. The results have implications for understanding both the initiation and affinity maturation of the immune response.
Figures
Similar articles
-
Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation.Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3327-32. doi: 10.1073/pnas.0511315103. Epub 2006 Feb 21. Proc Natl Acad Sci U S A. 2006. PMID: 16492756 Free PMC article.
-
B cell ligand discrimination through a spreading and contraction response.Science. 2006 May 5;312(5774):738-41. doi: 10.1126/science.1123940. Science. 2006. PMID: 16675699
-
B cells acquire antigen from target cells after synapse formation.Nature. 2001 May 24;411(6836):489-94. doi: 10.1038/35078099. Nature. 2001. PMID: 11373683
-
Viewing the antigen-induced initiation of B-cell activation in living cells.Immunol Rev. 2008 Feb;221:64-76. doi: 10.1111/j.1600-065X.2008.00583.x. Immunol Rev. 2008. PMID: 18275475 Review.
-
Antigen presentation by B lymphocytes: mechanisms and functional significance.Semin Immunol. 1989 Sep;1(1):5-12. Semin Immunol. 1989. PMID: 15630954 Review.
Cited by
-
A Rough Energy Landscape to Describe Surface-Linked Antibody and Antigen Bond Formation.Sci Rep. 2016 Oct 12;6:35193. doi: 10.1038/srep35193. Sci Rep. 2016. PMID: 27731375 Free PMC article.
-
Germinal Center B Cell Dynamics.Immunity. 2016 Sep 20;45(3):471-482. doi: 10.1016/j.immuni.2016.09.001. Immunity. 2016. PMID: 27653600 Free PMC article. Review.
-
Pivotal role for αV integrins in sustained Tfh support of the germinal center response for long-lived plasma cell generation.Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4462-4470. doi: 10.1073/pnas.1809329116. Epub 2019 Feb 15. Proc Natl Acad Sci U S A. 2019. PMID: 30770452 Free PMC article.
-
What counts in the immunological synapse?Mol Cell. 2014 Apr 24;54(2):255-62. doi: 10.1016/j.molcel.2014.04.001. Mol Cell. 2014. PMID: 24766889 Free PMC article. Review.
-
WASp Is Crucial for the Unique Architecture of the Immunological Synapse in Germinal Center B-Cells.Front Cell Dev Biol. 2021 Jun 14;9:646077. doi: 10.3389/fcell.2021.646077. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34195186 Free PMC article.
References
-
- Adorini L., Guery, J.C., Fuchs, S., Ortiz, N.V., Hammerling, G.J. and Momburg, F. (1993) Processing of endogenously synthesized hen egg-white lysozyme retained in the endoplasmic reticulum or in secretory form gives rise to a similar but not identical set of epitopes recognized by class II-restricted T cells. J. Immunol., 151, 3576–3586. - PubMed
-
- Batista F.D and Neuberger, M.S. (1998) Affinity dependence of the B cell response to antigen: a threshold, a ceiling and the importance of off-rate. Immunity, 8, 751–759. - PubMed
-
- Daeron M. (1997) Fc receptor biology. Annu. Rev. Immunol., 15, 203–234. - PubMed
-
- Davies D.R. and Padlan, E.A. (1990) Antibody–antigen complexes. Annu. Rev. Biochem., 59, 439–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

