C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B
- PMID: 10675330
- PMCID: PMC305599
- DOI: 10.1093/emboj/19.4.612
C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B
Abstract
Dominantly inherited multiple endocrine neoplasia type 2B (MEN2B) is characterized by tumors of the thyroid C-cells and adrenal chromaffin cells, together with ganglioneuromas of the gastrointestinal tract and other developmental abnormalities. Most cases are caused by substitution of threonine for Met918 in the RET receptor tyrosine kinase, which is believed to convert the RET gene to an oncogene by altering the enzyme's substrate specificity. We report the production of a mouse model of MEN2B by introduction of the corresponding mutation into the ret gene. Mutant mice displayed C-cell hyperplasia and chromaffin cell hyperplasia progressing to pheochromocytoma. Homozygotes did not develop gastrointestinal ganglioneuromas, but displayed ganglioneuromas of the adrenal medulla, enlargement of the associated sympathetic ganglia and a male reproductive defect. Surprisingly, homozygotes did not display any developmental defects attributable to a loss-of-function mutation. Thus, while our results support the conclusion that the Met918Thr substitution is responsible for MEN2B, they suggest that the substrate specificity of the RET kinase does not interfere with its normal role in the development of the kidneys and enteric nervous system.
Figures

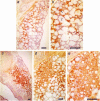

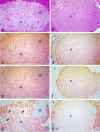

Similar articles
-
Ganglioneuromas and renal anomalies are induced by activated RET(MEN2B) in transgenic mice.Oncogene. 1999 Jan 28;18(4):877-86. doi: 10.1038/sj.onc.1202376. Oncogene. 1999. PMID: 10023663
-
Rudolf-Virchow-Preis 1995. The role of RET proto-oncogene mutation analysis in the diagnosis of multiple endocrine neoplasia type 2 (MEN 2) gene carriers and in the discrimination of sporadic and familial medullary thyroid carcinomas and pheochromocytomas.Verh Dtsch Ges Pathol. 1995;79:L-LV. Verh Dtsch Ges Pathol. 1995. PMID: 8600671
-
RET(Men2B)-transgene produces sympathoadrenal tumors but does not prevent intestinal aganglionosis in gdnf-/- or gfr alpha-1(-/-) mice.Pediatr Dev Pathol. 2001 Sep-Oct;4(5):446-53. doi: 10.1007/s10024001-0039-9. Pediatr Dev Pathol. 2001. PMID: 11779046
-
Mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2.Cancer Surv. 1995;25:195-205. Cancer Surv. 1995. PMID: 8718519 Review. No abstract available.
-
Multiple endocrine neoplasia type 2A. Study of a family.Rev Port Cardiol. 2000 Jan;19(1):11-31. Rev Port Cardiol. 2000. PMID: 10731788 Review. English, Portuguese.
Cited by
-
Molecular genetic alterations in adrenal and extra-adrenal pheochromocytomas and paragangliomas.Endocr Pathol. 2003 Winter;14(4):329-50. doi: 10.1385/ep:14:4:329. Endocr Pathol. 2003. PMID: 14739490 Review.
-
Constitutive Ret signaling leads to long-lasting expression of amphetamine-induced place conditioning via elevation of mesolimbic dopamine.Neuropharmacology. 2018 Jan;128:221-230. doi: 10.1016/j.neuropharm.2017.10.010. Epub 2017 Oct 13. Neuropharmacology. 2018. PMID: 29031851 Free PMC article.
-
Mouse models of thyroid cancer: A 2015 update.Mol Cell Endocrinol. 2016 Feb 5;421:18-27. doi: 10.1016/j.mce.2015.06.029. Epub 2015 Jun 27. Mol Cell Endocrinol. 2016. PMID: 26123589 Free PMC article. Review.
-
Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity.Nat Rev Cancer. 2014 Feb;14(2):108-19. doi: 10.1038/nrc3648. Epub 2014 Jan 20. Nat Rev Cancer. 2014. PMID: 24442145 Review.
-
Chronic 2-Fold Elevation of Endogenous GDNF Levels Is Safe and Enhances Motor and Dopaminergic Function in Aged Mice.Mol Ther Methods Clin Dev. 2020 Apr 11;17:831-842. doi: 10.1016/j.omtm.2020.04.003. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32368564 Free PMC article.
References
-
- Airaksinen M.S., Titievsky, A. and Saarma, M. (1999) GDNF family neurotrophic factor signaling: four masters, one servant?Mol. Cell. Neurosci., 13, 313–325. - PubMed
-
- Bocciardi R., Mograbi, B., Pasini, B., Borrello, M.G., Pierotti, M.A., Bourget, I., Fischer, S., Romeo, G. and Rossi, B. (1997) The multiple endocrine neoplasia type 2B point mutation switches the specificity of the Ret tyrosine kinase towards cellular substrates that are susceptible to interact with Crk and Nck. Oncogene, 15, 2257–2265. - PubMed
-
- Borrello M.G., et al. (1995)RET activation by germline MEN2A and MEN2B mutations. Oncogene, 11, 2419–2427. - PubMed
-
- Carlson K.M., Dou,S., Chi,D., Scavarda,N., Toshima,K., Jackson,C.E., Wells,S.A., Goodfellow,P.J. and Donis-Keller,H. (1994) Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc. Natl Acad. Sci. USA, 91, 1579–1583. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

