Regulation of E2F1 activity by acetylation
- PMID: 10675335
- PMCID: PMC305604
- DOI: 10.1093/emboj/19.4.662
Regulation of E2F1 activity by acetylation
Abstract
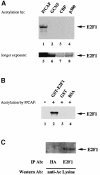
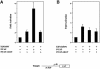
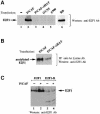
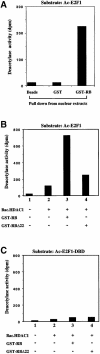
During the G(1) phase of the cell cycle, an E2F-RB complex represses transcription, via the recruitment of histone deacetylase activity. Phosphorylation of RB at the G(1)/S boundary generates a pool of 'free' E2F, which then stimulates transcription of S-phase genes. Given that E2F1 activity is stimulated by p300/CBP acetylase and repressed by an RB-associated deacetylase, we asked if E2F1 was subject to modification by acetylation. We show that the p300/CBP-associated factor P/CAF, and to a lesser extent p300/CBP itself, can acetylate E2F1 in vitro and that intracellular E2F1 is acetylated. The acetylation sites lie adjacent to the E2F1 DNA-binding domain and involve lysine residues highly conserved in E2F1, 2 and 3. Acetylation by P/CAF has three functional consequences on E2F1 activity: increased DNA-binding ability, activation potential and protein half-life. These results suggest that acetylation stimulates the functions of the non-RB bound 'free' form of E2F1. Consistent with this, we find that the RB-associated histone deacetylase can deacetylate E2F1. These results identify acetylation as a novel regulatory modification that stimulates E2F1's activation functions.
Figures




References
-
- Allen K.E., de la Luna, S., Kerkhoven, R.M., Bernards, R. and La Thangue, N.B. (1997) Distinct mechanisms of nuclear accumulation regulate the functional consequence of E2F transcription factors. J. Cell Sci., 110, 2819–2831. - PubMed
-
- Bagchi S., Weinmann, R. and Raychaudhuri, P. (1991) The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell, 65, 1063–1072. - PubMed
-
- Bannister A.J. and Kouzarides, T. (1996) The CBP co-activator is a histone acetyltransferase. Nature, 384, 641–643. - PubMed
-
- Boyes J., Byfield, P., Nakatani, Y. and Ogryzko, V. (1998) Regulation of activity of the transcription factor GATA-1 by acetylation. Nature, 396, 594–598. - PubMed
-
- Brehm A., Miska, E.A., McCance, D.J., Reid, J.L., Bannister, A.J. and Kouzarides, T. (1998) Retinoblastoma protein recruits deacetylase to repress transcription. Nature, 391, 597–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

