The basic helix-loop-helix transcription factor, dHAND, is required for vascular development
- PMID: 10675351
- PMCID: PMC377450
- DOI: 10.1172/JCI8856
The basic helix-loop-helix transcription factor, dHAND, is required for vascular development
Abstract
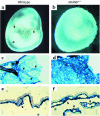
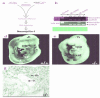
Reciprocal interactions between vascular endothelial cells and vascular mesenchymal cells are essential for angiogenesis. Here we show that the basic helix-loop-helix transcription factor, dHAND/Hand2, is expressed in the developing vascular mesenchyme and its derivative, vascular smooth muscle cells (VSMCs). Targeted deletion of the dHAND gene in mice revealed severe defects of embryonic and yolk sac vascular development by embryonic day 9.5. Vascular endothelial cells expressed most markers of differentiation. Vascular mesenchymal cells migrated appropriately but failed to make contact with vascular endothelial cells and did not differentiate into VSMCs. In a screen for genes whose expression was dependent upon dHAND (using subtractive hybridization comparing wild-type and dHAND-null hearts), the VEGF(165) receptor, neuropilin-1, was found to be downregulated in dHAND mutants. These results suggest that dHAND is required for vascular development and regulates angiogenesis, possibly through a VEGF signaling pathway.
Figures




References
-
- Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. - PubMed
-
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. - PubMed
-
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. - PubMed
-
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. - PubMed
-
- Dumont DJ, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

