Differential immune responses to alpha-gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation
- PMID: 10675356
- PMCID: PMC377438
- DOI: 10.1172/JCI7358
Differential immune responses to alpha-gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation
Abstract
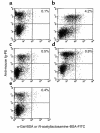
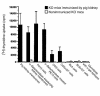
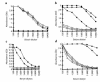
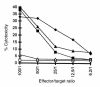
Xenograft recipients produce large amounts of high-affinity anti-Gal IgG in response to Galalpha1-3Galbeta1- 4GlcNAc-R (alpha-gal) epitopes on the graft. In contrast, ABO-mismatched allograft recipients undergo "accommodation," a state of very weak immune response to ABO antigens. These differences in anti-carbohydrate immune response were studied in alpha1,3galactosyltransferase knock-out mice. Pig kidney membranes administered to these mice elicited extensive production of anti-Gal IgG, whereas allogeneic kidney membranes expressing alpha-gal epitopes elicited only a weak anti-Gal IgM response. Anti-Gal IgG response to xenograft membranes depended on helper T cell activation and was inhibited by anti-CD40L antibody. These T cells were activated by xenopeptides and not by alpha-gal epitopes. Moreover, allogeneic cell membranes manipulated to express xenoproteins also induced anti-Gal IgG response. Xenoglycoproteins with alpha-gal epitopes are processed by anti-Gal B cells. Xenopeptides presented by these cells activate a large repertoire of helper T cells required for the differentiation of anti-Gal B cells into cells secreting anti-Gal IgG. Alloglycoproteins with alpha- gal epitopes have very few immunogenic peptides and fail to activate helper T cells. Similarly, ineffective helper T-cell activation prevents a strong immune response to blood group antigens in ABO-mismatched allograft recipients, thus enabling the development of accommodation.
Figures



References
-
- Galili U. Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–482. - PubMed
-
- Good AH, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. - PubMed
-
- Cozzi, E., et al. 1997. Effect of transgenic expression of human decay-accelerating factor on the inhibition of hyper acute rejection of pig organs. In Xenotransplantation. 2nd edition. D.K.C. Cooper, E. Kemp, J.L. Platt, and D.J.G. White, editors. Springer. Heidelberg, Germany. 665–682.
-
- Byrne GW, et al. Transgenic pigs expressing human CD59 and decay accelerating factor produce an intrinsic barrier to complement mediated damage. Transplantation. 1997;63:149–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

