Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis
- PMID: 10676659
- PMCID: PMC4469333
Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis
Abstract
Phage that display a surface peptide with the NGR sequence motif home selectively to tumor vasculature in vivo. A drug coupled to an NGR peptide has more potent antitumor effects than the free drug [W. Arap et al., Science (Washington DC), 279: 377-380, 1998]. We show here that the receptor for the NGR peptides in tumor vasculature is aminopeptidase N (APN; also called CD13). NGR phage specifically bound to immunocaptured APN and to cells engineered to express APN on their surface. Antibodies against APN inhibited in vivo tumor homing by the NGR phage. Immunohistochemical staining showed that APN expression is up-regulated in endothelial cells within mouse and human tumors. In another tissue that undergoes angiogenesis, corpus luteum, blood vessels also expressed APN, but APN was not detected in blood vessels of various other normal tissues stained under the same conditions. APN antagonists specifically inhibited angiogenesis in chorioallantoic membranes and in the retina and suppressed tumor growth. Thus, APN is involved in angiogenesis and can serve as a target for delivering drugs into tumors and for inhibiting angiogenesis.
Figures
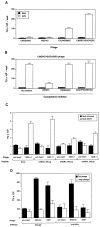
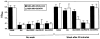


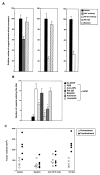
References
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
-
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. - PubMed
-
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrins. Science (Washington DC) 1995;270:1500–1502. - PubMed
-
- Hammes HP, Brownlee M, Jonczyk A, Sutter A, Preissner KT. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med. 1996;2:529–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
