Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry
- PMID: 10677424
- PMCID: PMC58868
- DOI: 10.1104/pp.122.2.295
Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry
Abstract
Tolerance of anoxia in maize root tips is greatly improved when seedlings are pretreated with 2 to 4 h of hypoxia. We describe the patterns of protein synthesis during hypoxic acclimation and anoxia. We quantified the incorporation of [(35)S]methionine into total protein and 262 individual proteins under different oxygen tensions. Proteins synthesized most rapidly under normoxic conditions continued to account for most of the proteins synthesized during hypoxic acclimation, while the production of a very few proteins was selectively enhanced. When acclimated root tips were placed under anoxia, protein synthesis was depressed and no "new" proteins were detected. We present evidence that protein synthesis during acclimation, but not during subsequent anoxia, is crucial for acclimation. The complex and quantitative changes in protein synthesis during acclimation necessitate identification of large numbers of individual proteins. We show that mass spectrometry can be effectively used to identify plant proteins arrayed by two-dimensional gel electrophoresis. Of the 48 protein spots analyzed, 46 were identified by matching to the protein database. We describe the expression of proteins involved in a wide range of cellular functions, including previously reported anaerobic proteins, and discuss their possible roles in adaptation of plants to low-oxygen stress.
Figures





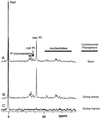
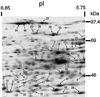

References
-
- Bandaranayake H, Esen A. Nucleotide sequence of a beta-glucosidase (glu2) cDNA from maize (accession no. U44087) (PGR 96-009) Plant Physiol. 1996;110:1048.
-
- Biemann K. Nomenclature for peptide fragment ions (positive ions) Methods Enzymol. 1990;193:886–887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

