The subcellular localization of acetyl-CoA carboxylase 2
- PMID: 10677481
- PMCID: PMC26453
- DOI: 10.1073/pnas.97.4.1444
The subcellular localization of acetyl-CoA carboxylase 2
Abstract
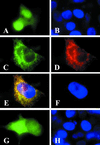
Animals, including humans, express two isoforms of acetyl-CoA carboxylase (EC ), ACC1 (M(r) = 265 kDa) and ACC2 (M(r) = 280 kDa). The predicted amino acid sequence of ACC2 contains an additional 136 aa relative to ACC1, 114 of which constitute the unique N-terminal sequence of ACC2. The hydropathic profiles of the two ACC isoforms generally are comparable, except for the unique N-terminal sequence in ACC2. The sequence of amino acid residues 1-20 of ACC2 is highly hydrophobic, suggesting that it is a leader sequence that targets ACC2 for insertion into membranes. The subcellular localization of ACC2 in mammalian cells was determined by performing immunofluorescence microscopic analysis using affinity-purified anti-ACC2-specific antibodies and transient expression of the green fluorescent protein fused to the C terminus of the N-terminal sequences of ACC1 and ACC2. These analyses demonstrated that ACC1 is a cytosolic protein and that ACC2 was associated with the mitochondria, a finding that was confirmed further by the immunocolocalization of a known human mitochondria-specific protein and the carnitine palmitoyltransferase 1. Based on analyses of the fusion proteins of ACC-green fluorescent protein, we concluded that the N-terminal sequences of ACC2 are responsible for mitochondrial targeting of ACC2. The association of ACC2 with the mitochondria is consistent with the hypothesis that ACC2 is involved in the regulation of mitochondrial fatty acid oxidation through the inhibition of carnitine palmitoyltransferase 1 by its product malonyl-CoA.
Figures


References
-
- Wakil S J. Am J Chem Soc. 1958;80:6465.
-
- Nugteren D H. Biochim Biophys Acta. 1965;106:280–290. - PubMed
-
- Hopwood D A, Sherman D H. Annu Rev Genet. 1990;24:37–66. - PubMed
-
- Thampy K G. J Biol Chem. 1989;264:17631–17634. - PubMed
-
- Bianchi A, Evans J L, Iverson A J, Nordlund A-C, Watts T D, Witters L A. J Biol Chem. 1990;265:1502–1509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

