A structural basis for integrin activation by the cytoplasmic tail of the alpha IIb-subunit
- PMID: 10677482
- PMCID: PMC26454
- DOI: 10.1073/pnas.040548197
A structural basis for integrin activation by the cytoplasmic tail of the alpha IIb-subunit
Abstract
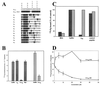
A key step in the activation of heterodimeric integrin adhesion receptors is the transmission of an agonist-induced cellular signal from the short alpha- and/or beta-cytoplasmic tails to the extracellular domains of the receptor. The structural details of how the cytoplasmic tails mediate such an inside-out signaling process remain unclear. We report herein the NMR structures of a membrane-anchored cytoplasmic tail of the alpha(IIb)-subunit and of a mutant alpha(IIb)-cytoplasmic tail that renders platelet integrin alpha(IIb)beta(3) constitutively active. The structure of the wild-type alpha(IIb)-cytoplasmic tail reveals a "closed" conformation where the highly conserved N-terminal membrane-proximal region forms an alpha-helix followed by a turn, and the acidic C-terminal loop interacts with the N-terminal helix. The structure of the active mutant is significantly different, having an "open" conformation where the interactions between the N-terminal helix and C-terminal region are abolished. Consistent with these structural differences, the two peptides differ in function: the wild-type peptide suppressed alpha(IIb)beta(3) activation, whereas the mutant peptide did not. These results provide an atomic explanation for extensive biochemical/mutational data and support a conformation-based "on/off switch" model for integrin activation.
Figures


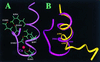

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

