Identification of the proton pathway in bacterial reaction centers: replacement of Asp-M17 and Asp-L210 with asn reduces the proton transfer rate in the presence of Cd2+
- PMID: 10677498
- PMCID: PMC26472
- DOI: 10.1073/pnas.97.4.1548
Identification of the proton pathway in bacterial reaction centers: replacement of Asp-M17 and Asp-L210 with asn reduces the proton transfer rate in the presence of Cd2+
Abstract
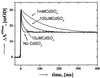
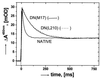
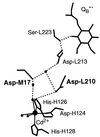
The reaction center (RC) from Rhodobacter sphaeroides converts light into chemical energy through the reduction and protonation of a bound quinone molecule Q(B) (the secondary quinone electron acceptor). We investigated the proton transfer pathway by measuring the proton-coupled electron transfer, k(AB)((2)) [Q(A)Q(B) + H(+) --> Q(A)(Q(B)H)(-)] in native and mutant RCs in the absence and presence of Cd(2+). Previous work has shown that the binding of Cd(2+) decreases k(AB)((2)) in native RCs approximately 100-fold. The preceding paper shows that bound Cd(2+) binds to Asp-H124, His-H126, and His-H128. This region represents the entry point for protons. In this work we investigated the proton transfer pathway connecting the entry point with Q(B) by searching for mutations that greatly affect k(AB)((2)) ( greater, similar10-fold) in the presence of Cd(2+), where k(AB)((2)) is limited by the proton transfer rate (k(H)). Upon mutation of Asp-L210 or Asp-M17 to Asn, k(H) decreased from approximately 60 s(-1) to approximately 7 s(-1), which shows the important role that Asp-L210 and Asp-M17 play in the proton transfer chain. By comparing the rate of proton transfer in the mutants (k(H) approximately 7 s(-1)) with that in native RCs in the absence of Cd(2+) (k(H) >/= 10(4) s(-1)), we conclude that alternate proton transfer pathways, which have been postulated, are at least 10(3)-fold less effective.
Figures

Similar articles
-
Identification of the proton pathway in bacterial reaction centers: cooperation between Asp-M17 and Asp-L210 facilitates proton transfer to the secondary quinone (QB).Biochemistry. 2001 Jun 12;40(23):6893-902. doi: 10.1021/bi010280a. Biochemistry. 2001. PMID: 11389604
-
Simultaneous replacement of Asp-L210 and Asp-M17 with Asn increases proton uptake by Glu-L212 upon first electron transfer to QB in reaction centers from Rhodobacter sphaeroides.Biochemistry. 2001 Nov 20;40(46):13826-32. doi: 10.1021/bi011423w. Biochemistry. 2001. PMID: 11705371
-
Coupling of electron transfer to proton uptake at the Q(B) site of the bacterial reaction center: a perspective from FTIR difference spectroscopy.Biochim Biophys Acta. 2008 Oct;1777(10):1229-48. doi: 10.1016/j.bbabio.2008.06.012. Epub 2008 Jul 11. Biochim Biophys Acta. 2008. PMID: 18671937 Review.
-
Mechanism of proton transfer inhibition by Cd(2+) binding to bacterial reaction centers: determination of the pK(A) of functionally important histidine residues.Biochemistry. 2003 Aug 19;42(32):9626-32. doi: 10.1021/bi0346648. Biochemistry. 2003. PMID: 12911304
-
Proton transfer pathways and mechanism in bacterial reaction centers.FEBS Lett. 2003 Nov 27;555(1):45-50. doi: 10.1016/s0014-5793(03)01149-9. FEBS Lett. 2003. PMID: 14630317 Review.
Cited by
-
Comparison of proton transfer paths to the QA and QB sites of the Rb. sphaeroides photosynthetic reaction centers.Photosynth Res. 2022 May;152(2):153-165. doi: 10.1007/s11120-022-00906-x. Epub 2022 Mar 28. Photosynth Res. 2022. PMID: 35344134
-
Mechanism of the formation of proton transfer pathways in photosynthetic reaction centers.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2103203118. doi: 10.1073/pnas.2103203118. Proc Natl Acad Sci U S A. 2021. PMID: 34301911 Free PMC article.
-
Correlation between C═O Stretching Vibrational Frequency and pKa Shift of Carboxylic Acids.J Phys Chem B. 2022 Jul 14;126(27):4999-5006. doi: 10.1021/acs.jpcb.2c02193. Epub 2022 Jun 28. J Phys Chem B. 2022. PMID: 35763701 Free PMC article.
-
Multiple scattering x-ray absorption studies of Zn2+ binding sites in bacterial photosynthetic reaction centers.Biophys J. 2005 Mar;88(3):2038-46. doi: 10.1529/biophysj.104.050971. Epub 2004 Dec 21. Biophys J. 2005. PMID: 15613631 Free PMC article.
-
Determination of the binding sites of the proton transfer inhibitors Cd2+ and Zn2+ in bacterial reaction centers.Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1542-7. doi: 10.1073/pnas.97.4.1542. Proc Natl Acad Sci U S A. 2000. PMID: 10677497 Free PMC article.
References
-
- Feher G, Allen J P, Okamura M Y, Rees D C. Nature (London) 1989;339:111–116.
-
- Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic Photosynthetic Bacteria. Dordrecht, the Netherlands: Kluwer; 1995.
-
- Graige M S, Paddock M L, Bruce J M, Feher G, Okamura M Y. J Am Chem Soc. 1996;118:9005–9016.
-
- Crofts A R, Wraight C A. Biochim Biophys Acta. 1983;726:149–185.
-
- McPherson P H, Okamura M Y, Feher G. Biochim Biophys Acta. 1990;1016:289–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

