Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity
- PMID: 10677537
- PMCID: PMC26516
- DOI: 10.1073/pnas.97.4.1802
Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity
Abstract
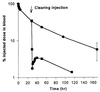
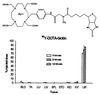
A covalent conjugate (NR-LU-10/SA) was prepared between streptavidin (SA) and NR-LU-10, a mAb that binds an antigen expressed on the surface of most human carcinomas. NR-LU-10/SA was injected into nude mice bearing human tumor xenografts. Injection of biotinylated galactosyl-human serum albumin reduced the circulating levels of conjugate by 95%. Subsequent administration of (90)Y-1,4,7, 10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-biotin achieved peak uptake at the tumor within 2 hr while >80% of the radioactivity was eliminated in the urine. A single dose of 600-800 microCi of (90)Y-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-biotin produced cures in 10/10 mice with established (>200 mm(3)) s.c. human small cell lung or colon cancer xenografts and 8/10 cures in mice with human breast cancer xenografts without significant toxicity.
Figures



References
-
- Langmuir V K. Nucl Med Biol. 1992;19:213–225. - PubMed
-
- Vittetta E S, Fulton R J, May R D, Till M, Uhr J W. Science. 1987;238:1098–1104. - PubMed
-
- Trail P A, Willner D, Lasch S J, Henderson A J, Hofstead S, Casazza A M, Firestone R A, Hellstrom I, Hellstrom K E. Science. 1993;261:212–215. - PubMed
-
- Jain R K. Sci Am. 1994;271:58–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

