Eukaryotic cell uptake of heparin-coated microspheres: a model of host cell invasion by Chlamydia trachomatis
- PMID: 10678910
- PMCID: PMC97251
- DOI: 10.1128/IAI.68.3.1080-1085.2000
Eukaryotic cell uptake of heparin-coated microspheres: a model of host cell invasion by Chlamydia trachomatis
Abstract
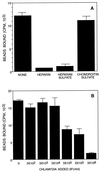
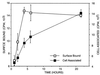
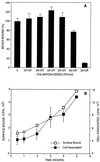
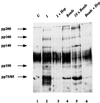
Using polystyrene microspheres coated with heparin or heparan sulfate, it was shown that coated microspheres specifically bound eukaryotic cells and were endocytosed by nonprofessional phagocytic cells. Coated microspheres displayed properties of binding to eukaryotic cells that were similar to those of chlamydiae, and the microspheres were competitively inhibited by chlamydial organisms. Endocytosis of heparin-coated beads resulted in the tyrosine phosphorylation of a similar set of host proteins as did endocytosis of chlamydiae; however, unlike viable chlamydial organisms, which prevent phagolysosomal fusion, endocytosed beads were trafficked to a lysosomal compartment. These findings suggest that heparin-coated beads and Chlamydia trachomatis enter eukaryotic cells by similar pathways.
Figures

Similar articles
-
Structural requirements of heparin binding to Chlamydia trachomatis.J Biol Chem. 1996 May 10;271(19):11134-40. J Biol Chem. 1996. PMID: 8626658
-
A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells.Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11143-8. doi: 10.1073/pnas.93.20.11143. Proc Natl Acad Sci U S A. 1996. PMID: 8855323 Free PMC article.
-
Chlamydia trachomatis glycosaminoglycan-dependent and independent attachment to eukaryotic cells.Microb Pathog. 1997 Jan;22(1):23-30. doi: 10.1006/mpat.1996.0087. Microb Pathog. 1997. PMID: 9032759
-
Molecular mimicry and Chlamydia trachomatis infection of eukaryotic cells.Trends Microbiol. 1994 Mar;2(3):99-101. doi: 10.1016/0966-842x(94)90543-6. Trends Microbiol. 1994. PMID: 8156278 Review.
-
Pathogenic Puppetry: Manipulation of the Host Actin Cytoskeleton by Chlamydia trachomatis.Int J Mol Sci. 2019 Dec 21;21(1):90. doi: 10.3390/ijms21010090. Int J Mol Sci. 2019. PMID: 31877733 Free PMC article. Review.
Cited by
-
Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate.Infect Immun. 2001 Feb;69(2):968-76. doi: 10.1128/IAI.69.2.968-976.2001. Infect Immun. 2001. PMID: 11159992 Free PMC article.
-
Mutagenesis and functional reconstitution of chlamydial major outer membrane proteins: VS4 domains are not required for pore formation but modify channel function.Infect Immun. 2001 Mar;69(3):1671-8. doi: 10.1128/IAI.69.3.1671-1678.2001. Infect Immun. 2001. PMID: 11179342 Free PMC article.
-
Heparin stimulates Staphylococcus aureus biofilm formation.Infect Immun. 2005 Aug;73(8):4596-606. doi: 10.1128/IAI.73.8.4596-4606.2005. Infect Immun. 2005. PMID: 16040971 Free PMC article.
References
-
- Andersson K, Carballeira N, Magnusson K E, Persson C, Stendahl O, Wolf-Watz H, Fallman M. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol Microbiol. 1996;20:1057–1069. - PubMed
-
- Bacallao R, Stelzer E H. Preservation of biological specimens for observation in a confocal fluorescence microscope and operational principals of confocal fluorescence microscopy. Methods Cell Biol. 1989;31:437–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

