Synthetic peptide immunogens elicit polyclonal and monoclonal antibodies specific for linear epitopes in the D motifs of Staphylococcus aureus fibronectin-binding protein, which are composed of amino acids that are essential for fibronectin binding
- PMID: 10678920
- PMCID: PMC97261
- DOI: 10.1128/IAI.68.3.1156-1163.2000
Synthetic peptide immunogens elicit polyclonal and monoclonal antibodies specific for linear epitopes in the D motifs of Staphylococcus aureus fibronectin-binding protein, which are composed of amino acids that are essential for fibronectin binding
Abstract
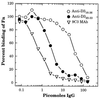
A fibronectin (Fn)-binding adhesin of Staphylococcus aureus contains three tandem 37- or 38-amino-acid motifs (D1, D2, and D3), which function to bind Fn. Plasma from patients with S. aureus infections contain antibodies that preferentially recognize ligand induced binding sites in the D motifs and do not inhibit Fn binding (F. Casolini, L. Visai, D. Joh, P. G. Conaldi, A. Toniolo, M. Höök, and P. Speziale, Infect. Immun. 66:5433-5442, 1998). To eliminate the influence of Fn binding on antibody development, we used synthetic peptide immunogens D1(21-34) and D3(20-33), which each contain a conserved pattern of amino acids that is essential for Fn binding but which cannot bind Fn without N- or C-terminal extensions. The D3(20-33) immunogen promoted the production of polyclonal antibodies that were 10-fold more effective as inhibitors of Fn-binding to the D3 motif than antibodies obtained by immunizing with an extended peptide D3(16-36), which exhibits functional Fn binding. The D3(20-33) immunogen also facilitated the production of a monoclonal antibody, 9C3, which was highly specific for the epitope SVDFEED, and abolished Fn binding by the D3 motif. When mixed with polyclonal anti-D1(21-34) immunoglobulin G, 70% inhibition of Fn binding to the three tandem D motifs was achieved compared to no more than 30% inhibition with either antibody preparation alone. Therefore, by immunizing with short synthetic peptides that are unable to bind Fn, we have effectively stimulated the production of antibodies specific for epitopes comprised of amino acids that are essential for Fn binding. Although these epitopes occur within a conserved pattern of amino acids that is required for Fn binding, the antibodies recognized specific linear epitope sequences and not a conserved structure common to all repeated motifs.
Figures


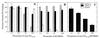

References
-
- Appel R D, Bairoch A, Hochstrasser D F. A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem Sci. 1994;19:258–260. - PubMed
-
- Archer G L. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis. 1998;26:1179–1181. - PubMed
-
- Balaban N, Goldkorn T, Nhan R T, Dang L B, Scott S, Ridgley R M, Rasooly A, Wright S C, Larrick J W, Rasooly R, Carlson J R. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science. 1998;280:438–440. - PubMed
-
- Brennan F R, Jones T D, Longstaff M, Chapman S, Bellaby T, Smith H, Xu F, Hamilton W D, Flock J I. Immunogenicity of peptides derived from a fibronectin-binding protein of S. aureus expressed on two different plant viruses. Vaccine. 1999;17:1846–1857. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

