The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species
- PMID: 10678965
- PMCID: PMC97306
- DOI: 10.1128/IAI.68.3.1491-1497.2000
The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species
Abstract
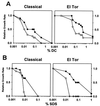
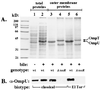
The transmembrane regulatory protein ToxR is required for expression of virulence factors in the human diarrheal pathogen Vibrio cholerae, including cholera toxin (CT) and the toxin coregulated pilus (TCP). ToxR is necessary for transcription of the gene encoding a second regulatory protein, ToxT, which is the direct transcriptional activator of CT and TCP genes. However, ToxR, independent of ToxT, directly activates and represses transcription of the outer membrane porins OmpU and OmpT, respectively. The genes encoding TCP and CT (and including ToxT) lie on horizontally acquired genetic elements, while the toxR, ompU, and ompT genes are apparently in the ancestral Vibrio chromosome. The contribution of ToxR-dependent modulation of outer membrane porins to cholera pathogenesis has remained unknown. We demonstrate that ToxR mediates enhanced bile resistance in a ToxT-independent manner. In both classical and El Tor biotypes of V. cholerae, a toxR mutant strain has a reduced minimum bactericidal concentration (MBC) of bile, the bile component deoxycholate (DC), and the anionic detergent sodium dodecyl sulfate (SDS) compared to both wild-type and toxT mutant strains. Classical and El Tor toxR mutant strains also exhibit reduced growth rates at subinhibitory concentrations of DC and SDS. Growth of either V. cholerae biotype in subinhibitory concentrations of bile or DC induces increased ToxR-dependent production of a major 38-kDa outer membrane protein, which was confirmed to be OmpU by Western blot. Measurement of transcription of a ompUp-lacZ fusion in both biotypes reveals stimulation (about two- to threefold) of ToxR-dependent ompU transcription by the presence of bile or DC, suggesting that ToxR may respond to the presence of bile. The toxR mutant strains of three additional human intestinal pathogenic Vibrio species, V. mimicus, V. fluvialis, and V. parahaemolyticus, display lower MBCs of bile, DC, and SDS and have altered outer membrane protein profiles compared to the parental wild-type strains. Our results demonstrate a conserved role for ToxR in the modulation of outer membrane proteins and bile resistance of pathogenic Vibrio species and suggest that these ToxR-dependent outer membrane proteins may mediate enhanced resistance to bile. We speculate that ToxR-mediated bile resistance was an early step in the evolution of V. cholerae as an intestinal pathogen.
Figures



References
-
- Blake P A. Historical perspectives on pandemic cholera. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C.: American Society for Microbiology; 1994. pp. 293–295.
-
- Carroll P A, Tashima K T, Rogers M B, DiRita V J, Calderwood S B. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol. 1997;25:1099–1111. - PubMed
-
- Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. - PubMed
-
- Crawford J A, Kaper J B, DiRita V J. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol Microbiol. 1998;29:235–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

