Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody
- PMID: 10678984
- PMCID: PMC97325
- DOI: 10.1128/IAI.68.3.1649-1654.2000
Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody
Abstract
We previously reported that a liposome-mannan vaccine (L-mann) of Candida albicans induces production of mouse antibodies that protect against disseminated candidiasis and vaginal infection. Immunoglobulin M (IgM) monoclonal antibody (MAb) B6.1, specific for a C. albicans cell surface beta-1,2-mannotriose, protects mice against both infections. Another IgM MAb, termed B6, which is specific for a different cell surface mannan epitope, does not protect against disseminated candidiasis. The B6.1 epitope is displayed homogeneously over the entire cell surface, compared to a patchy distribution of the B6 epitope. To determine if protection is restricted to an IgM class of antibody, we tested an IgG antibody. MAb C3.1 was obtained from L-mann-immunized mice. By results of sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, capture enzyme-linked immunosorbent assay, and immunodiffusion tests, MAb C3.1 is an IgG3 isotype. By epitope inhibition assays, we determined that MAb C3.1 is specific for same mannotriose as MAb B6. 1. As expected by the results of the inhibition assays, immunofluorescence microscopy showed that the C3.1 epitope is distributed on the yeast cell surface in a pattern identical to that of the B6.1 epitope. Kidney CFU and mean survival times of infected mice pretreated with MAb C3.1 indicated that the antibody enhanced resistance of mice against disseminated candidiasis. Mice in pseudoestrus that were given MAb C3.1 prior to vaginal infection developed fewer vaginal Candida CFU than control animals that received buffered saline instead of the antibody. The finding that an IgG3 antibody is protective is consistent with our hypothesis that epitope specificity and complement activation are related to the ability of an antibody to protect against candidiasis.
Figures

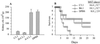

References
-
- Anacker R L, List R H, Mann R E, Hayes S F, Thomas L A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985;151:1052–1060. - PubMed
-
- Andrew S M, Titus J A. Purification and fragmentation of antibodies. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. I. New York, N.Y: John Wiley & Sons, Inc.; 1994. p. 2.7.4.
-
- Barclay G R, Yap P L, McClelland D B L, Jones R J, Roe E A, McCann M C, Micklem L R, James K. Characterisation of mouse monoclonal antibodies produced by immunisation with a single serotype component of a polyvalent Pseudomonas aeruginosa vaccine. J Med Microbiol. 1986;21:87–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

