Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract
- PMID: 10678986
- PMCID: PMC97327
- DOI: 10.1128/IAI.68.3.1664-1671.2000
Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract
Abstract
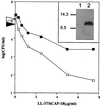
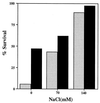
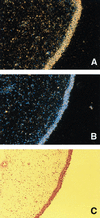
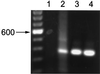
A number of pathogens of the upper respiratory tract express an unusual prokaryotic structure, phosphorylcholine (ChoP), on their cell surface. We tested the hypothesis that ChoP, also found on host membrane lipids in the form of phosphatidylcholine, acts so as to decrease killing by antimicrobial peptides that target differences between bacterial and host membranes. In Haemophilus influenzae, ChoP is a phase-variable structure on the oligosaccharide portion of the lipopolysaccharide (LPS). There was a bactericidal effect of the peptide LL-37/hCAP18 on a nontypeable H. influenzae strain, with an increasing selection for the ChoP(+) phase as the concentration of the peptide was raised from 0 to 10 microgram/ml. Moreover, constitutive ChoP-expressing mutants of unrelated strains showed up to 1,000-fold-greater survival compared to mutants without ChoP. The effect of ChoP on resistance to killing by LL-37/hCAP18 was dependent on the salt concentration and was observed only when bacteria were grown in the presence of environmental choline, a requirement for the expression of ChoP on the LPS. Further studies established that there is transcription of the LL-37/hCAP18 gene on the epithelial surface of the human nasopharynx in situ and inducible transcription in epithelial cells derived from the upper airway. The presence of highly variable amounts of LL-37/hCAP18 in normal nasal secretions (<1.2 to >80 microgram/ml) was demonstrated with an antibody against this peptide. It was concluded that ChoP alters the bacterial cell surface so as mimic host membrane lipids and decrease killing by LL-37/hCAP18, an antimicrobial peptide that may be expressed on the mucosal surface of the nasopharynx in bactericidal concentrations.
Figures




Similar articles
-
Phase variation with altering phosphorylcholine expression of nontypeable Haemophilus influenzae affects bacteria clearance and mucosal immune response in the middle ear and nasopharynx.Auris Nasus Larynx. 2021 Feb;48(1):57-64. doi: 10.1016/j.anl.2020.07.001. Epub 2020 Jul 17. Auris Nasus Larynx. 2021. PMID: 32684402
-
Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori.Gastroenterology. 2003 Dec;125(6):1613-25. doi: 10.1053/j.gastro.2003.08.028. Gastroenterology. 2003. PMID: 14724813
-
Proinflammatory Cytokines Impair Vitamin D-Induced Host Defense in Cultured Airway Epithelial Cells.Am J Respir Cell Mol Biol. 2017 Jun;56(6):749-761. doi: 10.1165/rcmb.2016-0289OC. Am J Respir Cell Mol Biol. 2017. PMID: 28231019
-
The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections.Peptides. 2010 Sep;31(9):1791-8. doi: 10.1016/j.peptides.2010.06.016. Epub 2010 Jun 25. Peptides. 2010. PMID: 20600427 Review.
-
The biosynthesis and role of phosphorylcholine in pathogenic and nonpathogenic bacteria.Trends Microbiol. 2023 Jul;31(7):692-706. doi: 10.1016/j.tim.2023.01.006. Epub 2023 Feb 28. Trends Microbiol. 2023. PMID: 36863982 Free PMC article. Review.
Cited by
-
ChoK-ing the Pathogenic Bacteria: Potential of Human Choline Kinase Inhibitors as Antimicrobial Agents.Biomed Res Int. 2020 Jul 9;2020:1823485. doi: 10.1155/2020/1823485. eCollection 2020. Biomed Res Int. 2020. PMID: 32695809 Free PMC article. Review.
-
Choline Kinase Emerges as a Promising Drug Target in Gram-Positive Bacteria.Front Microbiol. 2019 Oct 15;6:2146. doi: 10.3389/fmicb.2019.02146. eCollection 2019. Front Microbiol. 2019. PMID: 31681254 Free PMC article.
-
Antimicrobial peptides: current status and therapeutic potential.Drugs. 2003;63(4):389-406. doi: 10.2165/00003495-200363040-00005. Drugs. 2003. PMID: 12558461 Review.
-
Modulation of Haemophilus influenzae interaction with hydrophobic molecules by the VacJ/MlaA lipoprotein impacts strongly on its interplay with the airways.Sci Rep. 2018 May 2;8(1):6872. doi: 10.1038/s41598-018-25232-y. Sci Rep. 2018. PMID: 29720703 Free PMC article.
-
Effects of Surface Charge of Amphiphilic Peptides on Peptide-Lipid Interactions in the Gas Phase and in Solution.Anal Chem. 2025 Mar 18;97(10):5808-5817. doi: 10.1021/acs.analchem.5c00283. Epub 2025 Mar 7. Anal Chem. 2025. PMID: 40052744 Free PMC article.
References
-
- Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1999.
-
- Boman H. Antibacterial peptides: key components needed in immunity. Cell. 1991;65:205–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

