Rab7: a key to lysosome biogenesis
- PMID: 10679007
- PMCID: PMC14786
- DOI: 10.1091/mbc.11.2.467
Rab7: a key to lysosome biogenesis
Abstract
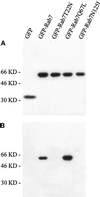
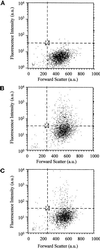
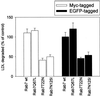
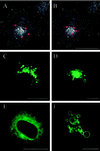
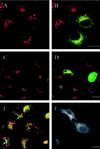
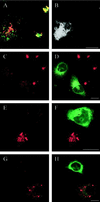
The molecular machinery behind lysosome biogenesis and the maintenance of the perinuclear aggregate of late endocytic structures is not well understood. A likely candidate for being part of this machinery is the small GTPase Rab7, but it is unclear whether this protein is associated with lysosomes or plays any role in the regulation of the perinuclear lysosome compartment. Previously, Rab7 has mainly been implicated in transport from early to late endosomes. We have now used a new approach to analyze the role of Rab7: transient expression of Enhanced Green Fluorescent Protein (EGFP)-tagged Rab7 wt and mutant proteins in HeLa cells. EGFP-Rab7 wt was associated with late endocytic structures, mainly lysosomes, which aggregated and fused in the perinuclear region. The size of the individual lysosomes as well as the degree of perinuclear aggregation increased with the expression levels of EGFP-Rab7 wt and, more dramatically, the active EGFP-Rab7Q67L mutant. In contrast, upon expression of the dominant-negative mutants EGFP-Rab7T22N and EGFP-Rab7N125I, which localized mainly to the cytosol, the perinuclear lysosome aggregate disappeared and lysosomes, identified by colocalization of cathepsin D and lysosome-associated membrane protein-1, became dispersed throughout the cytoplasm, they were inaccessible to endocytosed molecules such as low-density lipoprotein, and their acidity was strongly reduced, as determined by decreased accumulation of the acidotropic probe LysoTracker Red. In contrast, early endosomes associated with Rab5 and the transferrin receptor, late endosomes enriched in the cation-independent mannose 6-phosphate receptor, and the trans-Golgi network, identified by its enrichment in TGN-38, were unchanged. These data demonstrate for the first time that Rab7, controlling aggregation and fusion of late endocytic structures/lysosomes, is essential for maintenance of the perinuclear lysosome compartment.
Figures

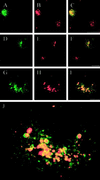
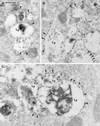
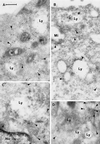
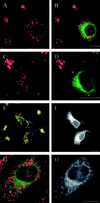
References
-
- Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are reformed from the resultant hybrid organelles. J Cell Sci. 1997;110:2027–2040. - PubMed
-
- Brown MS, Goldstein JL. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975;6:307–316. - PubMed
-
- Bucci C, Lütcke A, Steele-Mortimer O, Chiariello M, Olkkonen VM, Dupree P, Bruni CB, Simons K, Zerial M. Cooperative regulation of endocytosis by three rab5 isoforms. FEBS Lett. 1995;366:65–71. - PubMed
-
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. - PubMed
-
- Carlsson SR, Roth J, Piller F, Fukuda M. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2: major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem. 1988;263:18911–18919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

