Occludin 1B, a variant of the tight junction protein occludin
- PMID: 10679019
- PMCID: PMC14798
- DOI: 10.1091/mbc.11.2.627
Occludin 1B, a variant of the tight junction protein occludin
Abstract
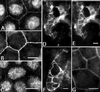
Occludin and claudin are the major integral membrane components of the mammalian tight junction. Although more than 11 distinct claudins have been identified, only 1 occludin transcript has been reported thus far. Therefore, we searched by reverse transcription-PCR for occludin-related sequences in Madin-Darby canine kidney (MDCK) mRNA and identified a transcript encoding an alternatively spliced form of occludin, designated occludin 1B. The occludin 1B transcript contained a 193-base pair insertion encoding a longer form of occludin with a unique N-terminal sequence of 56 amino acids. Analysis of the MDCK occludin gene revealed an exon containing the 193-base pair sequence between the exons encoding the original N terminus and the distal sequence, suggesting that occludin and occludin 1B arise from alternative splicing of one transcript. To assess the expression and distribution of occludin 1B, an antibody was raised against its unique N-terminal domain. Immunolabeling of occludin 1B in MDCK cells revealed a distribution indistinguishable from that of occludin. Furthermore, occludin 1B staining at cell-to-cell contacts was also found in cultured T84 human colon carcinoma cells and in frozen sections of mouse intestine. Immunoblots of various mouse tissues revealed broad coexpression of occludin 1B with occludin. The wide epithelial distribution and the conservation across species suggests a potentially important role for occludin 1B in the structure and function of the tight junction.
Figures



References
-
- Balda MS, Anderson JM, Matter K. The SH3 domain of the tight junction protein ZO-1 binds to a serine protein kinase that phosphorylates a region C-terminal to this domain. FEBS Lett. 1996a;399:326–332. - PubMed
-
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996b;134:1031–1049. - PMC - PubMed
-
- Cordenonsi M, Mazzon E, De Rigo L, Baraldo S, Meggio F, Citi S. Occludin dephosphorylation in early development of Xenopus laevis. J Cell Sci. 1997;110:3131–3139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

