Carrier-mediated delivery of 9-(2-phosphonylmethoxyethyl)adenine to parenchymal liver cells: a novel therapeutic approach for hepatitis B
- PMID: 10681306
- PMCID: PMC89714
- DOI: 10.1128/AAC.44.3.477-483.2000
Carrier-mediated delivery of 9-(2-phosphonylmethoxyethyl)adenine to parenchymal liver cells: a novel therapeutic approach for hepatitis B
Abstract
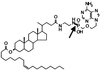
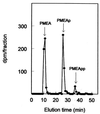
Our aim is to selectively deliver 9-(2-phosphonylmethoxyethyl)adenine (PMEA) to parenchymal liver cells, the primary site of hepatitis B virus (HBV) infection. Selective delivery is necessary because PMEA, which is effective against HBV in vitro, is hardly taken up by the liver in vivo. Lactosylated reconstituted high-density lipoprotein (LacNeoHDL), a lipid particle that is specifically internalized by parenchymal liver cells via the asialoglycoprotein receptor, was used as the carrier. PMEA could be incorporated into the lipid moiety of LacNeoHDL by attaching, via an acid-labile bond, lithocholic acid-3alpha-oleate to the drug. The uptake of the lipophilic prodrug (PMEA-LO) by the liver was substantially increased after incorporation into LacNeoHDL. Thirty minutes after injection of [(3)H]PMEA-LO-loaded LacNeoHDL into rats, the liver contained 68.9% +/- 7.7% of the dose (free [(3)H]PMEA, <5%). Concomitantly, the uptake by the kidney was reduced to <2% of the dose (free [(3)H]PMEA, >45%). The hepatic uptake of PMEA-LO-loaded LacNeoHDL occurred mainly by parenchymal cells (88.5% +/- 8.2% of the hepatic uptake). Moreover, asialofetuin inhibited the liver association by >75%, indicating uptake via the asialoglycoprotein receptor. The acid-labile linkage in PMEA-LO, designed to release PMEA during lysosomal processing of the prodrug-loaded carrier, was stable at physiological pH but was hydrolyzed at lysosomal pH (half-life, 60 to 70 min). Finally, subcellular fractionation indicates that the released PMEA is translocated to the cytosol, where it is converted into its active diphosphorylated metabolite. In conclusion, lipophilic modification and incorporation of PMEA into LacNeoHDL improves the biological fate of the drug and may lead to an enhanced therapeutic efficacy against chronic hepatitis B.
Figures



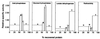
References
-
- Aduma P, Connelly M C, Srinivas R V, Fridland A. Metabolic diversity and antiviral activities of acyclic nucleoside phosphonates. Mol Pharmacol. 1995;47:816–822. - PubMed
-
- Annaert P, Van Gelder J, Naesens L, De Clercq E, Van den Mooter G, Kinget R, Augustijns P. Carrier mechanisms involved in the transepithelial transport of bis(POM)-PMEA and its metabolites across Caco-2 monolayers. Pharm Res. 1998;15:1168–1173. - PubMed
-
- Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Ann Rev Biochem. 1982;51:531–554. - PubMed
-
- Balzarini J, Hatse S, Naesens L, De Clercq E. Selection and characterization of murine leukemia L1210 cells with high-level resistance to the cytostatic activity of the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl)adenine (PMEA) Biochim Biophys Acta. 1998;1402:29–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

