A critical residue for isoform difference in tetrodotoxin affinity is a molecular determinant of the external access path for local anesthetics in the cardiac sodium channel
- PMID: 10681444
- PMCID: PMC15800
- DOI: 10.1073/pnas.030438797
A critical residue for isoform difference in tetrodotoxin affinity is a molecular determinant of the external access path for local anesthetics in the cardiac sodium channel
Abstract
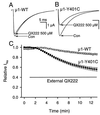
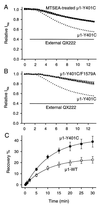
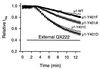
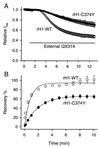
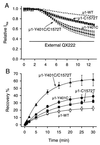
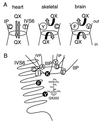
Membrane-impermeant quaternary derivatives of lidocaine (QX222 and QX314) block cardiac Na(+) channels when applied from either side of the membrane, but they block neuronal and skeletal muscle channels poorly from the outside. To find the molecular determinants of the cardiac external QX access path, mutations of adult rat skeletal muscle (micro1) and rat heart (rH1) Na(+) channels were studied by two-electrode voltage clamp in Xenopus oocytes. Mutating the micro1 domain I P-loop Y401, which is the critical residue for isoform differences in tetrodotoxin block, to the heart sequence (Y401C) allowed outside QX222 block, but its mutation to brain type (Y401F) showed little block. mu1-Y401C accelerated recovery from block by internal QX222. Block by external QX222 in mu1-Y401C was diminished by chemical modification with methanethiosulfonate ethylammonium (MTSEA) to the outer vestibule or by a double mutant (mu1-Y401C/F1579A), which altered the putative local anesthetic binding site. The reverse mutation in heart rH1-C374Y reduced outside QX314 block and slowed dissociation of internal QX222. Mutation of mu1-C1572 in IVS6 to Thr, the cardiac isoform residue (C1572T), allowed external QX222 block, and accelerated recovery from internal QX222 block, as reported. Blocking efficacy of outside QX222 in mu1-Y401C was more than that in mu1-C1572T, and the double mutant (mu1-Y401C/C1572T) accelerated internal QX recovery more than mu1-Y401C or mu1-C1572T alone. We conclude that the isoform-specific residue (Tyr/Phe/Cys) in the P-loop of domain I plays an important role in drug access as well as in tetrodotoxin binding. Isoform-specific residues in the IP-loop and IVS6 determine outside drug access to an internal binding site.
Figures

Similar articles
-
Structural and gating changes of the sodium channel induced by mutation of a residue in the upper third of IVS6, creating an external access path for local anesthetics.Mol Pharmacol. 2001 Apr;59(4):684-91. doi: 10.1124/mol.59.4.684. Mol Pharmacol. 2001. PMID: 11259611
-
Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+ channel.Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11839-43. doi: 10.1073/pnas.92.25.11839. Proc Natl Acad Sci U S A. 1995. PMID: 8524860 Free PMC article.
-
Sodium channel selectivity filter regulates antiarrhythmic drug binding.Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):14126-31. doi: 10.1073/pnas.94.25.14126. Proc Natl Acad Sci U S A. 1997. PMID: 9391164 Free PMC article.
-
The enigmatic drug binding site for sodium channel inhibitors.Curr Mol Pharmacol. 2010 Nov;3(3):129-44. doi: 10.2174/1874467211003030129. Curr Mol Pharmacol. 2010. PMID: 20565383 Review.
-
The tetrodotoxin binding site is within the outer vestibule of the sodium channel.Mar Drugs. 2010 Feb 1;8(2):219-34. doi: 10.3390/md8020219. Mar Drugs. 2010. PMID: 20390102 Free PMC article. Review.
Cited by
-
Gene Conversion Facilitates the Adaptive Evolution of Self-Resistance in Highly Toxic Newts.Mol Biol Evol. 2021 Sep 27;38(10):4077-4094. doi: 10.1093/molbev/msab182. Mol Biol Evol. 2021. PMID: 34129031 Free PMC article.
-
Structural basis for antiarrhythmic drug interactions with the human cardiac sodium channel.Proc Natl Acad Sci U S A. 2019 Feb 19;116(8):2945-2954. doi: 10.1073/pnas.1817446116. Epub 2019 Feb 6. Proc Natl Acad Sci U S A. 2019. PMID: 30728299 Free PMC article.
-
Molecular architecture of the voltage-dependent Na channel: functional evidence for alpha helices in the pore.J Gen Physiol. 2001 Aug;118(2):171-82. doi: 10.1085/jgp.118.2.171. J Gen Physiol. 2001. PMID: 11479344 Free PMC article.
-
Pharmacological properties of neuronal TTX-resistant sodium channels and the role of a critical serine pore residue.Pflugers Arch. 2005 Dec;451(3):454-63. doi: 10.1007/s00424-005-1463-x. Epub 2005 Jun 25. Pflugers Arch. 2005. PMID: 15981012
-
Accessibility of mid-segment domain IV S6 residues of the voltage-gated Na+ channel to methanethiosulfonate reagents.J Physiol. 2004 Dec 1;561(Pt 2):403-13. doi: 10.1113/jphysiol.2004.067579. Epub 2004 Oct 7. J Physiol. 2004. PMID: 15579536 Free PMC article.
References
-
- Catterall W A. Physiol Rev. 1992;72:S15–S48. - PubMed
-
- Fozzard H A, Hanck D A. Physiol Rev. 1996;76:887–926. - PubMed
-
- Terlau H, Heinemann S H, Stühmer W, Pusch M, Conti F, Imoto K, Numa S. FEBS Lett. 1991;293:93–96. - PubMed
-
- Chiamvimonvat N, Pérez-García M T, Ranjan R, Marban E, Tomaselli G F. Neuron. 1996;16:1037–1047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

