Transferrin receptor 2: continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis
- PMID: 10681454
- PMCID: PMC15780
- DOI: 10.1073/pnas.040548097
Transferrin receptor 2: continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis
Abstract
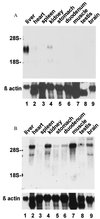
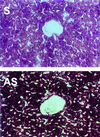
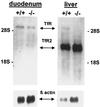
Hereditary hemochromatosis (HH) is a common autosomal recessive disorder characterized by excess absorption of dietary iron and progressive iron deposition in several tissues, particularly liver. Liver disease resulting from iron toxicity is the major cause of death in HH. Hepatic iron loading in HH is progressive despite down-regulation of the classical transferrin receptor (TfR). Recently a human cDNA highly homologous to TfR was identified and reported to encode a protein (TfR2) that binds holotransferrin and mediates uptake of transferrin-bound iron. We independently identified a full-length murine EST encoding the mouse orthologue of the human TfR2. Although homologous to murine TfR in the coding region, the TfR2 transcript does not contain the iron-responsive elements found in the 3' untranslated sequence of TfR mRNA. To determine the potential role for TfR2 in iron uptake by liver, we investigated TfR and TfR2 expression in normal mice and murine models of dietary iron overload (2% carbonyl iron), dietary iron deficiency (gastric parietal cell ablation), and HH (HFE -/-). Northern blot analyses demonstrated distinct tissue-specific patterns of expression for TfR and TfR2, with TfR2 expressed highly only in liver where TfR expression is low. In situ hybridization demonstrated abundant TfR2 expression in hepatocytes. In contrast to TfR, TfR2 expression in liver was not increased in iron deficiency. Furthermore, hepatic expression of TfR2 was not down-regulated with dietary iron loading or in the HFE -/- model of HH. From these observations, we propose that TfR2 allows continued uptake of Tf-bound iron by hepatocytes even after TfR has been down-regulated by iron overload, and this uptake contributes to the susceptibility of liver to iron loading in HH.
Figures




References
-
- Bacon B R, Powell L W, Adams P C, Kresina T F, Hoofnagle J H. Gastroenterology. 1999;116:193–207. - PubMed
-
- Niederau C, Erhardt A, Häussinger D, Strohmeyer G. J Hepatol. 1999;30:6–11. - PubMed
-
- Bonkovsky H L. Am J Med Sci. 1991;301:32–43. - PubMed
-
- Hubert N, Lescoat G, Sciot R, Moirand R, Jego P, Leroyer P, Brissot P. J Hepatol. 1993;18:301–312. - PubMed
-
- Testa U, Pelosi E, Peschle C. Crit Rev Oncog. 1993;4:241–276. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

