Endodermal Nodal-related signals and mesoderm induction in Xenopus
- PMID: 10683171
- PMCID: PMC2292107
- DOI: 10.1242/dev.127.6.1173
Endodermal Nodal-related signals and mesoderm induction in Xenopus
Abstract
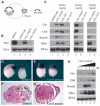
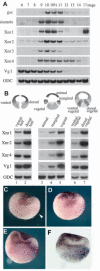
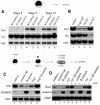
In Xenopus, mesoderm induction by endoderm at the blastula stage is well documented, but the molecular nature of the endogenous inductive signals remains unknown. The carboxy-terminal fragment of Cerberus, designated Cer-S, provides a specific secreted antagonist of mesoderm-inducing Xenopus Nodal-Related (Xnr) factors. Cer-S does not inhibit signalling by other mesoderm inducers such as Activin, Derrière, Vg1 and BMP4, nor by the neural inducer Xnr3. In the present study we show that Cer-S blocks the induction of both dorsal and ventral mesoderm in animal-vegetal Nieuwkoop-type recombinants. During blastula stages Xnr1, Xnr2 and Xnr4 are expressed in a dorsal to ventral gradient in endodermal cells. Dose-response experiments using cer-S mRNA injections support the existence of an endogenous activity gradient of Xnrs. Xnr expression at blastula can be activated by the vegetal determinants VegT and Vg1 acting in synergy with dorsal (beta)-catenin. The data support a modified model for mesoderm induction in Xenopus, in which mesoderm induction is mediated by a gradient of multiple Nodal-related signals released by endoderm at the blastula stage.
Figures




References
-
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 1997;68:45–57. - PubMed
-
- Bouwmeester T, Kim SH, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. - PubMed
-
- Boterenbrood EC, Nieuwkoop PD. The formation of the mesoderm in urodelean amphibians. V. Its regional induction by the endoderm. Wilhelm Roux’ Arch. Dev. Biol. 1973;173:319–332. - PubMed
-
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

