Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels
- PMID: 10683204
- PMCID: PMC1571891
- DOI: 10.1038/sj.bjp.0703102
Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels
Abstract
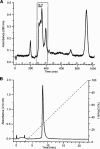
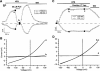
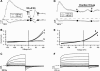
It was the aim of this study to look for a high-affinity and selective polypeptide toxin, which could serve as a probe for the volume-regulated anion channel (VRAC) or the calcium-activated chloride channel (CaCC). We have partially purified chlorotoxin, including new and homologous short chain insectotoxins, from the crude venom of Leiurus quinquestriatus quinquestriatus (Lqq) by means of gel filtration chromatography. Material eluting between 280 and 420 min, corresponding to fractions 15-21, was lyophilized and tested on VRAC and CaCC, using the whole-cell patch-clamp technique. We have also tested the commercially available chlorotoxin on VRAC, CaCC, the cystic fibrosis transmembrane conductance regulator (CFTR) and on the glioma specific chloride channel (GCC). VRAC and the correspondent current, I(Cl,swell), was activated in Cultured Pulmonary Artery Endothelial (CPAE) cells by a 25% hypotonic solution. Neither of the fractions 16-21 significantly inhibited I(Cl,swell) (n=4-5). Ca(2+)-activated Cl(-) currents, I(Cl,Ca), activated by loading T84 cells via the patch pipette with 1 microM free Ca(2+), were not inhibited by any of the tested fractions (15-21), (n=2-5). Chlorotoxin (625 nM) did neither effect I(Cl,swell) nor I(Cl,Ca) (n=4-5). The CFTR channel, transiently transfected in COS cells and activated by a cocktail containing IBMX and forskolin, was not affected by 1.2 microM chlorotoxin (n=5). In addition, it did not affect currents through GCC. We conclude that submicromolar concentrations of chlorotoxin do not block volume-regulated, Ca(2+)-activated and CFTR chloride channels and that it can not be classified as a general chloride channel toxin.
Figures
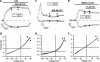


Similar articles
-
Inhibition of volume-regulated and calcium-activated chloride channels by the antimalarial mefloquine.J Pharmacol Exp Ther. 2000 Oct;295(1):29-36. J Pharmacol Exp Ther. 2000. PMID: 10991957
-
Block by fluoxetine of volume-regulated anion channels.Br J Pharmacol. 1999 Jan;126(2):508-14. doi: 10.1038/sj.bjp.0702314. Br J Pharmacol. 1999. PMID: 10077245 Free PMC article.
-
Inhibition by mibefradil, a novel calcium channel antagonist, of Ca(2+)- and volume-activated Cl- channels in macrovascular endothelial cells.Br J Pharmacol. 1997 Jun;121(3):547-55. doi: 10.1038/sj.bjp.0701140. Br J Pharmacol. 1997. PMID: 9179399 Free PMC article.
-
Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion.Dan Med Bull. 2010 Oct;57(10):B4191. Dan Med Bull. 2010. PMID: 21040688 Review.
-
Amazing chloride channels: an overview.Acta Physiol Scand. 2003 Feb;177(2):119-47. doi: 10.1046/j.1365-201X.2003.01060.x. Acta Physiol Scand. 2003. PMID: 12558550 Review.
Cited by
-
Characterization of a Family of Scorpion Toxins Modulating Ca2+-Activated Cl- Current in Vascular Myocytes.Toxins (Basel). 2022 Nov 10;14(11):780. doi: 10.3390/toxins14110780. Toxins (Basel). 2022. PMID: 36356031 Free PMC article.
-
Recent advances in diagnosis and treatment of gliomas using chlorotoxin-based bioconjugates.Am J Nucl Med Mol Imaging. 2014 Aug 15;4(5):385-405. eCollection 2014. Am J Nucl Med Mol Imaging. 2014. PMID: 25143859 Free PMC article. Review.
-
Current Biochemical Applications and Future Prospects of Chlorotoxin in Cancer Diagnostics and Therapeutics.Adv Pharm Bull. 2019 Oct;9(4):510-520. doi: 10.15171/apb.2019.061. Epub 2019 Oct 24. Adv Pharm Bull. 2019. PMID: 31857956 Free PMC article. Review.
-
Chlorotoxin-A Multimodal Imaging Platform for Targeting Glioma Tumors.Toxins (Basel). 2018 Nov 26;10(12):496. doi: 10.3390/toxins10120496. Toxins (Basel). 2018. PMID: 30486274 Free PMC article. Review.
-
In vivo bio-imaging using chlorotoxin-based conjugates.Curr Pharm Des. 2011 Dec;17(38):4362-71. doi: 10.2174/138161211798999375. Curr Pharm Des. 2011. PMID: 22204434 Free PMC article. Review.
References
-
- CUPPENS H., LIN W., JASPERS M., COSTES B., TENG H., VANKEERBERGHEN A., JORISSEN M., DROOGMANS G., REYNAERT I., GOOSSENS M., NILIUS B., CASSIMAN J.J. Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation. J. Clin. Invest. 1998;101:487–496. - PMC - PubMed
-
- DEBIN J.A., MAGGIO J.E., STRICHARTZ G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol. 1993;264:C361–C369. - PubMed
-
- DEBIN J.A., STRICHARTZ G.R. Chloride channel inhibition by the venom of the scorpion Leiurus quinquestriatus. Toxicon. 1991;29:1403–1408. - PubMed
-
- JENTSCH T.J., GÜNTHER W. Chloride Channels: an Emerging Molecular Picture. BioEssays. 1997;19:117–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

