Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification
- PMID: 10684251
- PMCID: PMC2169372
- DOI: 10.1083/jcb.148.4.691
Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification
Abstract
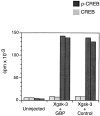
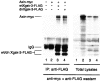
Glycogen synthase kinase 3 (GSK-3) is a constitutively active kinase that negatively regulates its substrates, one of which is beta-catenin, a downstream effector of the Wnt signaling pathway that is required for dorsal-ventral axis specification in the Xenopus embryo. GSK-3 activity is regulated through the opposing activities of multiple proteins. Axin, GSK-3, and beta-catenin form a complex that promotes the GSK-3-mediated phosphorylation and subsequent degradation of beta-catenin. Adenomatous polyposis coli (APC) joins the complex and downregulates beta-catenin in mammalian cells, but its role in Xenopus is less clear. In contrast, GBP, which is required for axis formation in Xenopus, binds and inhibits GSK-3. We show here that GSK-3 binding protein (GBP) inhibits GSK-3, in part, by preventing Axin from binding GSK-3. Similarly, we present evidence that a dominant-negative GSK-3 mutant, which causes the same effects as GBP, keeps endogenous GSK-3 from binding to Axin. We show that GBP also functions by preventing the GSK-3-mediated phosphorylation of a protein substrate without eliminating its catalytic activity. Finally, we show that the previously demonstrated axis-inducing property of overexpressed APC is attributable to its ability to stabilize cytoplasmic beta-catenin levels, demonstrating that APC is impinging upon the canonical Wnt pathway in this model system. These results contribute to our growing understanding of how GSK-3 regulation in the early embryo leads to regional differences in beta-catenin levels and establishment of the dorsal axis.
Figures
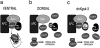
Similar articles
-
Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin.Mol Cell Biol. 1999 Oct;19(10):7147-57. doi: 10.1128/MCB.19.10.7147. Mol Cell Biol. 1999. PMID: 10490650 Free PMC article.
-
Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos.Mol Cell Biol. 1998 May;18(5):2867-75. doi: 10.1128/MCB.18.5.2867. Mol Cell Biol. 1998. PMID: 9566905 Free PMC article.
-
Relationship of vegetal cortical dorsal factors in the Xenopus egg with the Wnt/beta-catenin signaling pathway.Mech Dev. 1999 Dec;89(1-2):93-102. doi: 10.1016/s0925-4773(99)00210-5. Mech Dev. 1999. PMID: 10559484
-
Modulation of Wnt signaling by Axin and Axil.Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review.
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
-
The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling.Genes Dev. 2002 Aug 15;16(16):2073-84. doi: 10.1101/gad.230402. Genes Dev. 2002. PMID: 12183362 Free PMC article.
-
Wnt modulators in the biotech pipeline.Dev Dyn. 2010 Jan;239(1):102-14. doi: 10.1002/dvdy.22181. Dev Dyn. 2010. PMID: 20014100 Free PMC article. Review.
-
The maternally localized RNA fatvg is required for cortical rotation and germ cell formation.Mech Dev. 2007 May;124(5):350-63. doi: 10.1016/j.mod.2007.02.001. Epub 2007 Feb 21. Mech Dev. 2007. PMID: 17376659 Free PMC article.
-
Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT2 (nuclear factor of activated T cells 2).J Cell Physiol. 2012 Apr;227(4):1529-37. doi: 10.1002/jcp.22872. J Cell Physiol. 2012. PMID: 21678407 Free PMC article.
-
GSK3 beta N-terminus binding to p53 promotes its acetylation.Mol Cancer. 2009 Mar 5;8:14. doi: 10.1186/1476-4598-8-14. Mol Cancer. 2009. PMID: 19265551 Free PMC article.
References
-
- Behrens J., Jerchow B.A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kuhl M., Wedlich D., Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK-3β. Science. 1998;280:596–599. - PubMed
-
- Behrens J., von Kries J.P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
-
- Bhanot P., Brink M., Samos C., Hsieh J., Wang Y., Macke J., Andrew D., Nathans J., Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. - PubMed
-
- Bienz M. APCthe plot thickens. Curr. Opin. Genet. Dev. 1999;9:595–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

