Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery
- PMID: 10684252
- PMCID: PMC2169366
- DOI: 10.1083/jcb.148.4.703
Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery
Abstract
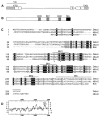
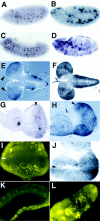
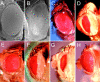
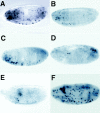
Bcl-2 family of proteins are key regulators of apoptosis. Both proapoptotic and antiapoptotic members of this family are found in mammalian cells, but no such proteins have been described in insects. Here, we report the identification and characterization of Debcl, the first Bcl-2 homologue in Drosophila melanogaster. Structurally, Debcl is similar to Bax-like proapoptotic Bcl-2 family members. Ectopic expression of Debcl in cultured cells and in transgenic flies causes apoptosis, which is inhibited by coexpression of the baculovirus caspase inhibitor P35, indicating that Debcl is a proapoptotic protein that functions in a caspase-dependent manner. debcl expression correlates with developmental cell death in specific Drosophila tissues. We also show that debcl genetically interacts with diap1 and dark, and that debcl-mediated apoptosis is not affected by gene dosage of rpr, hid, and grim. Biochemically, Debcl can interact with several mammalian and viral prosurvival Bcl-2 family members, but not with the proapoptotic members, suggesting that it may regulate apoptosis by antagonizing prosurvival Bcl-2 proteins. RNA interference studies indicate that Debcl is required for developmental apoptosis in Drosophila embryos. These results suggest that the main components of the mammalian apoptosis machinery are conserved in insects.
Figures



Comment in
-
Drosophila apoptosis and Bcl-2 genes: outliers fly in.J Cell Biol. 2000 Feb 21;148(4):625-7. doi: 10.1083/jcb.148.4.625. J Cell Biol. 2000. PMID: 10684245 Free PMC article. No abstract available.
References
-
- Abrams J.M. An emerging blueprint for apoptosis in Drosophila . Trends Cell Biol. 1999;9:435–440. - PubMed
-
- Abrams J.M., White K., Fessler L.I., Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117:29–43. - PubMed
-
- Adams J.M., Cory S. The Bcl-2 protein familyarbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- Antonsson B., Conti F., Ciavatta A., Montessuit S., Lewis S., Martinou I., Bernasconi L., Bernard A., Mermod J.J., Mazzei G. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. - PubMed
-
- Bhat M.A., Izaddoost S., Lu Y., Cho K.O., Choi K.W., Bellen H.J. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

