Infection of primary human monocytes by Epstein-Barr virus
- PMID: 10684275
- PMCID: PMC111749
- DOI: 10.1128/jvi.74.6.2612-2619.2000
Infection of primary human monocytes by Epstein-Barr virus
Abstract
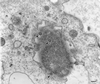
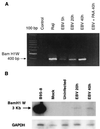
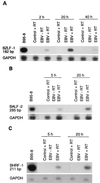
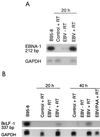
Previous studies have reported that infection of monocytes by viruses such as cytomegalovirus and human immunodeficiency virus weakens host natural immunity. In the present study, we demonstrated the capability of Epstein-Barr virus (EBV) to infect and replicate in freshly isolated human monocytes. Using electron microscopy analysis, we observed the presence of EBV virions in the cytoplasm and nuclei of approximately 20% of monocytes. This was confirmed by Southern blot analysis of EBV genomic DNA sequences in isolated nuclei from monocytes. Infection of monocytes by EBV leads to the activation of the replicative cycle. This was supported by the detection of immediate-early lytic mRNA BZLF-1 transcripts, and by the presence of two early lytic transcripts (BALF-2, which appears to function in DNA replication, and BHRF-1, also associated with the replicative cycle). The late lytic BcLF-1 transcripts, which code for the major nucleocapsid protein, were also detected, as well as EBNA-1 transcripts. However, attempts to detect EBNA-2 transcripts have yielded negative results. Viral replication was also confirmed by the release of newly synthesized infectious viral particles in supernatants of EBV-infected monocytes. EBV-infected monocytes were found to have significantly reduced phagocytic activity, as evaluated by the quantification of ingested carboxylated fluoresceinated latex beads. Taken together, our results suggest that EBV infection of monocytes and alteration of their biological functions might represent a new mechanism to disrupt the immune response and promote viral propagation during the early stages of infection.
Figures
References
-
- Adams D O, Hamilton T A. Macrophages as destructive cells in host defense. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles in clinical correlates. 2nd ed. New York, N.Y: Raven Press; 1992. pp. 637–662.
-
- Andoh A, Fujiyama Y, Kitoh K, Hodohara K, Bamba T, Hosoda S. Flow cytometric assay for phagocytosis of human monocytes mediated via Fcγ-receptors and complement receptor CR1 (CD35) Cytometry. 1991;12:677–686. - PubMed
-
- Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Séguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. - PubMed
-
- Beaulieu A D, Paquin R, Gosselin J. Epstein-Barr virus modulates de novo protein synthesis in human neutrophils. Blood. 1995;86:2789–2798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

