Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization
- PMID: 10684276
- PMCID: PMC111750
- DOI: 10.1128/jvi.74.6.2620-2627.2000
Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization
Abstract
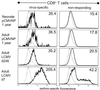
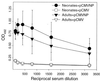
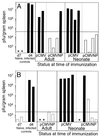
Virus infections are devastating to neonates, and the induction of active antiviral immunity in this age group is an important goal. Here, we show that a single neonatal DNA vaccination induces cellular and humoral immune responses which are maintained for a significant part of the animal's life span. We employ a sensitive technique which permits the first demonstration and quantitation, directly ex vivo, of virus-specific CD8(+) T cells induced by DNA immunization. One year postvaccination, antigen-specific CD8(+) T cells were readily detectable and constituted 0.5 to 1% of all CD8(+) T cells. By several criteria-including cytokine production, perforin content, development of lytic ability, and protective capacity-DNA vaccine-induced CD8(+) memory T cells were indistinguishable from memory cells induced by immunization with a conventional (live-virus) vaccine. Analyses of long-term humoral immune responses revealed that, in contrast to the strong immunoglobulin G2a (IgG2a) skewing of the humoral response seen after conventional vaccination, IgG1 and IgG2a levels were similar in DNA-vaccinated neonatal and adult animals, indicating a balanced T helper response. Collectively, these results show that a single DNA vaccination within hours or days of birth can induce long-lasting CD8(+) T- and B-cell responses; there is no need for secondary immunization (boosting). Furthermore, the observed immune responses induced in neonates and in adults are indistinguishable by several criteria, including protection against virus challenge.
Figures



References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Bachmann M F, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–299. - PubMed
-
- Barrios C, Brawand P, Berney M, Brandt C, Lambert P H, Siegrist C A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–1496. - PubMed
-
- Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine. 1998;16:1675–1682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

