Activation of lymphocyte signaling by the R1 protein of rhesus monkey rhadinovirus
- PMID: 10684288
- PMCID: PMC111762
- DOI: 10.1128/jvi.74.6.2721-2730.2000
Activation of lymphocyte signaling by the R1 protein of rhesus monkey rhadinovirus
Abstract
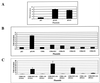
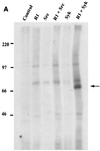
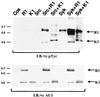
Rhesus monkey rhadinovirus (RRV) is a gamma-2 herpesvirus that exhibits a considerable degree of similarity to the human Kaposi's sarcoma-associated herpesvirus (KSHV). The R1 protein of RRV is distantly related to the K1 protein of KSHV, and R1, like K1, can contribute to cell growth transformation. In this study we analyzed the ability of the cytoplasmic tail of R1 to function as a signal transducer. The cytoplasmic domain of the R1 protein contains several tyrosine residues whose phosphorylation is induced in cells expressing Syk kinase. Expression of a CD8 chimera protein containing the extracellular and transmembrane domains of CD8 fused to the cytoplasmic domain of R1 mobilized intracellular calcium and induced cellular tyrosine phosphorylation in B cells upon stimulation with anti-CD8 antibody. None of the CD8-R1 cytoplasmic deletion mutants tested were able to mobilize intracellular calcium or to induce tyrosine phosphorylation to a significant extent upon addition of anti-CD8 antibody. Expression of wild-type R1 protein activated nuclear factor of activated T lymphocytes (NFAT) eightfold in B cells in the absence of antibody stimulation; expression of the CD8-R1C chimera strongly induced NFAT activity (60-fold) but only upon the addition of anti-CD8 antibody. We conclude that the cytoplasmic domain of R1 is capable of transducing signals that elicit B-lymphocyte activation events. The signal-inducing properties of R1 appear to be similar to those of K1 but differ in that the required sequences are distributed over a much longer stretch of the cytoplasmic domain (>150 amino acids). In addition, the induction of calcium mobilization was considerably longer in duration and stronger with R1 than with K1.
Figures









References
-
- Beaufils P, Choquet D, Mamoun R Z, Malissen B. The (YXXL/I)2 signaling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 1993;12:5105–5112. - PMC - PubMed
-
- Cambier J C. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. - PubMed
-
- Cambier J C, Jensen W A. The hetero-oligomeric antigen receptor complex and its coupling to cytoplasmic effectors. Curr Opin Genet Dev. 1994;4:55–63. - PubMed
-
- Cambier J C, Pleiman C M, Clark M R. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

