Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors
- PMID: 10684294
- PMCID: PMC111768
- DOI: 10.1128/jvi.74.6.2777-2785.2000
Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors
Abstract
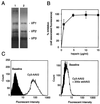
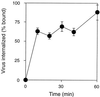
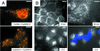
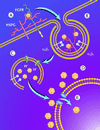
We have investigated the infectious entry pathway of adeno-associated virus (AAV) and recombinant AAV vectors by assessing AAV-mediated gene transfer and by covalently conjugating fluorophores to AAV and monitoring entry by fluorescence microscopy. We examined AAV entry in HeLa cells and in HeLa cell lines which inducibly expressed a dominant interfering mutant of dynamin. The data demonstrate that AAV internalizes rapidly by standard receptor-mediated endocytosis from clathrin-coated pits (half-time <10 min). The lysosomotropic agents ammonium chloride and bafilomycin A(1) prevent AAV-mediated gene transfer when present during the first 30 min after the onset of endocytosis, indicating that AAV escapes from early endosomes yet requires an acidic environment for penetration into the cytosol. Following release from the endosome, AAV rapidly moves to the cell nucleus and accumulates perinuclearly beginning within 30 min after the onset of endocytosis. We present data indicating that escape of AAV from the endosome and trafficking of viral particles to the nucleus are unaffected by the presence of adenovirus, the primary helper virus for a productive AAV infection. Within 2 h, viral particles could be detected within the cell nucleus, suggesting that AAV enters the nucleus prior to uncoating. Interestingly, the majority of the intracellular virus particles remain in a stable perinuclear compartment even though gene expression from nuclear AAV genomes can be detected. This suggests that the process of nuclear entry is rate limiting or that AAV entry involves multiple pathways. Nevertheless, these data establish specific points in the AAV infectious entry process and have allowed the generation of a model for future expansion to specific cell types and AAV vector analysis in vivo.
Figures




References
-
- Bartlett J S, Samulski R J. Fluorescent viral vectors: a new technique for the pharmacological analysis of gene therapy. Nat Med. 1998;4:635–637. - PubMed
-
- Bartlett J S, Samulski R J, McCown T J. Selective and rapid uptake of adeno-associated virus type-2 (AAV-2) in brain. Hum Gene Ther. 1998;9:1181–1186. - PubMed
-
- Basak S, Turner H, Parr S. Identification of a 40- to 42-kDa attachment polypeptide for canine parvovirus in A72 cells. Virology. 1994;205:7–16. - PubMed
-
- Basak S, Turner H. Infectious entry pathway for canine parvovirus. Virology. 1992;186:368–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

