Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer
- PMID: 10684306
- PMCID: PMC111780
- DOI: 10.1128/jvi.74.6.2888-2894.2000
Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer
Abstract
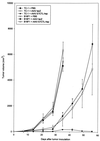
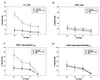
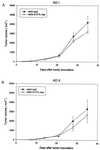
In this study, we explore a potential vaccine for human papillomavirus (HPV)-induced tumors, using heat shock protein as an adjuvant, a peptide vaccine for safety, and adeno-associated virus (AAV) as a gene delivery vector. The tumor vaccine was devised by constructing a chimeric gene which contained HPV type 16 E7 cytotoxic T-lymphocyte (CTL) epitope DNA (M. C. Feltkamp, H. L. Smits, M. P. Vierboom, R. P. Minnaar, B. M. de Jongh, J. W. Drijfhout, J. ter Schegget, C. J. Melief, and W. M. Kast, Eur. J. Immunol. 23:2242-2249, 1993) fused with the heat shock protein gene as a tumor vaccine delivered via AAV. Our results demonstrate that this vaccine can eliminate tumor cells in syngeneic animals and induce CD4- and CD8-dependent CTL activity in vitro. Moreover, studies with knockout mice with distinct T-cell deficiencies confirm that CTL-induced tumor protection is CD4 and CD8 dependent. Taken together, the evidence indicates that this chimeric gene delivered by AAV has potential as a cervical cancer vaccine.
Figures



Similar articles
-
Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16.J Immunol. 2001 Dec 1;167(11):6471-9. doi: 10.4049/jimmunol.167.11.6471. J Immunol. 2001. PMID: 11714814
-
Enhancement of Sindbis virus self-replicating RNA vaccine potency by linkage of Mycobacterium tuberculosis heat shock protein 70 gene to an antigen gene.J Immunol. 2001 May 15;166(10):6218-26. doi: 10.4049/jimmunol.166.10.6218. J Immunol. 2001. PMID: 11342644
-
Long-term protection against human papillomavirus e7-positive tumor by a single vaccination of adeno-associated virus vectors encoding a fusion protein of inactivated e7 of human papillomavirus 16/18 and heat shock protein 70.Hum Gene Ther. 2010 Jan;21(1):109-19. doi: 10.1089/hum.2009.139. Hum Gene Ther. 2010. PMID: 19715402
-
Human papillomavirus genotype 16 vaccines for cervical cancer prophylaxis and treatment.Curr Opin Oncol. 2000 Sep;12(5):466-73. doi: 10.1097/00001622-200009000-00014. Curr Opin Oncol. 2000. PMID: 10975555 Review.
-
Towards vaccines against human papillomavirus type-16 genital infections.Vaccine. 1993;11(6):603-11. doi: 10.1016/0264-410x(93)90302-e. Vaccine. 1993. PMID: 8391738 Review.
Cited by
-
Role of human papillomavirus and cell cycle-related variants in squamous cell carcinoma of the oropharynx.J Biomed Res. 2010 Sep;24(5):339-46. doi: 10.1016/S1674-8301(10)60047-4. J Biomed Res. 2010. PMID: 23554649 Free PMC article. No abstract available.
-
Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice.J Virol. 2007 Nov;81(21):11840-9. doi: 10.1128/JVI.01253-07. Epub 2007 Aug 22. J Virol. 2007. PMID: 17715240 Free PMC article.
-
Current state in the development of candidate therapeutic HPV vaccines.Expert Rev Vaccines. 2016 Aug;15(8):989-1007. doi: 10.1586/14760584.2016.1157477. Epub 2016 Mar 7. Expert Rev Vaccines. 2016. PMID: 26901118 Free PMC article. Review.
-
Preclinical Immunogenicity and Efficacy Studies for Therapeutic Vaccines for Human Papillomavirus-Type-16-Associated Cancer.Vaccines (Basel). 2024 Jun 4;12(6):616. doi: 10.3390/vaccines12060616. Vaccines (Basel). 2024. PMID: 38932345 Free PMC article.
-
Induction of CD8 T cells by vaccination with recombinant adenovirus expressing human papillomavirus type 16 E5 gene reduces tumor growth.J Virol. 2000 Oct;74(19):9083-9. doi: 10.1128/jvi.74.19.9083-9089.2000. J Virol. 2000. PMID: 10982354 Free PMC article.
References
-
- Borysiewicz L K, Fiander A, Nimako M, Man S, Wilkinson G W, Westmoreland D, Evans A S, Adams M, Stacey S N, Boursnell M E, Rutherford E, Hickling J K, Inglis S C. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. - PubMed
-
- Boursnell M E, Rutherford E, Hickling J K, Rollinson E A, Munro A J, Rolley N, McLean C S, Borysiewicz L K, Vousden K, Inglis S C. Construction and characterization of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine. 1996;14:1485–1494. - PMC - PubMed
-
- Brodsky J L. Post-translational protein translocation: not all hsc70s are created equal. Trends Biochem Sci. 1996;21:122–126. - PubMed
-
- Burghardt E, Webb M J, Monaghan J M, Kindermann G. Surgical gynecological oncology. New York, N.Y: Thieme Medical Publishers; 1993.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

