Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation
- PMID: 10684310
- PMCID: PMC111784
- DOI: 10.1128/jvi.74.6.2907-2912.2000
Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation
Abstract
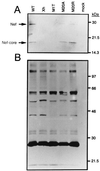
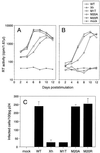
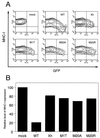
The N-terminal alpha-helix domain of the human immunodeficiency virus type 1 (HIV-1) Nef protein plays important roles in enhancement of viral infectivity, virion incorporation of Nef, and the down-regulation of major histocompatibility complex class I (MHC-I) expression on cell surfaces. In this study, we demonstrated that Met 20 in the alpha-helix domain was indispensable for the ability of Nef to modulate MHC-I expression but not for other events. We also showed that Met 20 was unnecessary for the down-regulation of CD4. These findings indicate that the region governing MHC-I down-regulation is proximate in the alpha-helix domain but is dissociated functionally from that determining enhancement of viral infectivity, virion incorporation of Nef, and CD4 down-regulation.
Figures


References
-
- Adachi A, Ono N, Sakai H, Ogawa K, Shibata R, Kiyomasu T, Masuike H, Ueda S. Generation and characterization of the human immunodeficiency virus type 1 mutants. Arch Virol. 1991;117:45–58. - PubMed
-
- Aiken C, Krause L, Chen Y, Trono D. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology. 1996;217:293–300. - PubMed
-
- Akari H, Uchiyama T, Fukumori T, Iida S, Koyama A H, Adachi A. Pseudotyping human immunodeficiency virus type 1 by vesicular stomatitis virus G protein does not reduce the cell-dependent requirement of Vif for optimal infectivity: functional difference between Vif and Nef. J Gen Virol. 1999;80:2945–2950. - PubMed
-
- Barnham K J, Monks S A, Hinds M G, Azad A A, Norton R S. Solution structure of a polypeptide from the N terminus of the HIV protein Nef. Biochemistry. 1997;36:5970–5980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

