Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells
- PMID: 10684851
- PMCID: PMC2195829
- DOI: 10.1084/jem.191.4.593
Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells
Abstract
Attachment of Helicobacter pylori to gastric epithelial cells induces various cellular responses, including the tyrosine phosphorylation of an unknown 145-kD protein and interleukin 8 production. Here we show that this 145-kD protein is the cagA product of H. pylori, an immunodominant, cytotoxin-associated antigen. Epithelial cells infected with various H. pylori clinical isolates resulted in generation of tyrosine-phosphorylated proteins ranging from 130 to 145 kD in size that were also induced in vitro by mixing host cell lysate with bacterial lysate. When epithelial cells were infected with [(35)S]methionine-labeled H. pylori, a radioactive 145-kD protein was detected in the immunoprecipitates with antiphosphotyrosine antibody or anti-CagA (cytotoxin-associated gene A) antibody. Consistently, the 145-kD protein recognized by the anti-CagA and antiphosphotyrosine antibodies was induced in epithelial cells after infection of wild-type H. pylori but not the cagA::Km mutant. Furthermore, the amino acid sequence of the phosphorylated 145-kD protein induced by H. pylori infection was identical to the H. pylori CagA sequence. These results reveal that the tyrosine-phosphorylated 145-kD protein is H. pylori CagA protein, which may be delivered from attached bacteria into the host cytoplasm. The identification of the tyrosine-phosphorylated protein will thus provide further insights into understanding the precise roles of CagA protein in H. pylori pathogenesis.
Figures
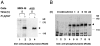
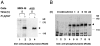








Comment in
-
Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell.J Exp Med. 2000 Feb 21;191(4):587-92. doi: 10.1084/jem.191.4.587. J Exp Med. 2000. PMID: 10684850 Free PMC article. Review. No abstract available.
References
-
- Marshall B.J., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. - PubMed
-
- Blaser M.J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 1990;161:626–633. - PubMed
-
- Parsonnet J., Friedman G.D., Vandersteen D.P., Chang Y., Vogelman J.H., Orentreich N., Sibley R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991;325:1127–1131. - PubMed
-
- Parsonnet J., Hansen S., Rodriguez L., Gelb A.B., Warnke R.A., Jellum E., Orentreich N., Vogelman J.H., Friedman G.D. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 1994;330:1267–1271. - PubMed

