Differential tumor surveillance by natural killer (NK) and NKT cells
- PMID: 10684858
- PMCID: PMC2195840
- DOI: 10.1084/jem.191.4.661
Differential tumor surveillance by natural killer (NK) and NKT cells
Abstract
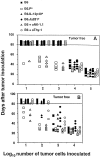
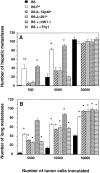
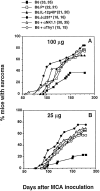
Natural tumor surveillance capabilities of the host were investigated in six different mouse tumor models where endogenous interleukin (IL)-12 does or does not dictate the efficiency of the innate immune response. Gene-targeted and lymphocyte subset-depleted mice were used to establish the relative importance of natural killer (NK) and NK1.1(+) T (NKT) cells in protection from tumor initiation and metastasis. In the models examined, CD3(-) NK cells were responsible for tumor rejection and protection from metastasis in models where control of major histocompatibility complex class I-deficient tumors was independent of IL-12. A protective role for NKT cells was only observed when tumor rejection required endogenous IL-12 activity. In particular, T cell receptor Jalpha281 gene-targeted mice confirmed a critical function for NKT cells in protection from spontaneous tumors initiated by the chemical carcinogen, methylcholanthrene. This is the first description of an antitumor function for NKT cells in the absence of exogenously administered potent stimulators such as IL-12 or alpha-galactosylceramide.
Figures



References
-
- Talmadge J.E., Meyers K.M., Prieur D.J., Starkey J.R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980;284:622–624. - PubMed
-
- Karre K., Ljunggren H.G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. - PubMed
-
- Salazar-Mather T.P., Ishikawa R., Biron C.A. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J. Immunol. 1996;157:3054–3064. - PubMed
-
- Bendelac A., Rivera M.N., Park S.H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

