Inhibition of interleukin 7 receptor signaling by antigen receptor assembly
- PMID: 10684865
- PMCID: PMC2195828
- DOI: 10.1084/jem.191.4.737
Inhibition of interleukin 7 receptor signaling by antigen receptor assembly
Abstract
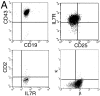
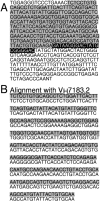
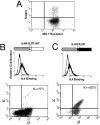
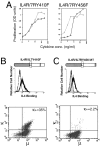
After the productive rearrangement of immunoglobulin (Ig) heavy chain genes, precursor (pre-)B lymphocytes undergo a limited number of cell divisions in response to interleukin (IL)-7. Here, we present evidence that this phase of IL-7-dependent expansion is constrained by an inhibitory signal initiated by antigen receptor assembly. A line of pre-B cells from normal murine bone marrow that expresses a mu heavy chain with a D-proximal V(H)7183.2 region divides continuously in IL-7. IL-7 responsiveness ceases upon differentiation to the mu(1), kappa(1) stage, despite continuing expression of the IL-7 receptor (IL-7R), suggesting that antigen receptor assembly inhibits IL-7 responsiveness. This is confirmed by introduction of a rearranged lambda light chain gene, which inhibits proliferative signaling through the IL-7R. Inhibition is specific to the IL-7R, because it is overcome by replacement of the IL-7R cytoplasmic domain with corresponding sequences from the closely related IL-2Rbeta chain. Alteration of a single tyrosine residue, Tyr410, in the IL-7R cytoplasmic domain to phenylalanine also prevents the inhibition of proliferation after antigen receptor assembly. Thus, the loss of IL-7 responsiveness after antigen receptor assembly may be mediated through the recruitment of an inhibitory molecule to this residue. Our findings identify a novel mechanism that limits cytokine-dependent proliferation during B lymphopoiesis. This mechanism may be essential for the proper regulation of peripheral B lymphocyte numbers.
Figures

Similar articles
-
Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression.J Immunol. 2000 Feb 15;164(4):1961-70. doi: 10.4049/jimmunol.164.4.1961. J Immunol. 2000. PMID: 10657646
-
Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression.J Immunol. 1998 Dec 1;161(11):6038-45. J Immunol. 1998. PMID: 9834086
-
Interleukin-7 signaling in human B cell precursor acute lymphoblastic leukemia cells and murine BAF3 cells involves activation of STAT1 and STAT5 mediated via the interleukin-7 receptor alpha chain.Leukemia. 1996 Aug;10(8):1317-25. Leukemia. 1996. PMID: 8709637
-
Role of interleukin-7 in T-cell development from hematopoietic stem cells.Immunol Rev. 1998 Oct;165:13-28. doi: 10.1111/j.1600-065x.1998.tb01226.x. Immunol Rev. 1998. PMID: 9850848 Review.
-
Fidelity and infidelity in commitment to B-lymphocyte lineage development.Immunol Rev. 2000 Jun;175:104-11. Immunol Rev. 2000. PMID: 10933595 Review.
Cited by
-
Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling.Nat Rev Immunol. 2009 Mar;9(3):195-205. doi: 10.1038/nri2491. Nat Rev Immunol. 2009. PMID: 19240758 Review.
-
Regulation of B cell fate, survival, and function by mitochondria and autophagy.Mitochondrion. 2018 Jul;41:58-65. doi: 10.1016/j.mito.2017.11.005. Epub 2017 Nov 23. Mitochondrion. 2018. PMID: 29175010 Free PMC article. Review.
-
Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling.Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15113-8. doi: 10.1073/pnas.2436348100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657335 Free PMC article.
References
-
- Goodwin R.G., Friend D., Ziegler S.F., Jerzy R., Falk B.A., Gimpel S., Cosman D., Dower S.K., March C.J., Namen A.E. Cloning of the human and murine interleukin-7 receptorsdemonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. - PubMed
-
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. - PubMed
-
- Noguchi M., Nakamura Y., Russell S.M., Ziegler S.F., Tsang M., Cao X., Leonard W.J. Interleukin-2 receptor gamma chaina functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. - PubMed
-
- Russell S.M., Keegan A.D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M.C., Miyajima A., Puri R.K., Paul W.E. Interleukin-2 receptor gamma chaina functional component of the interleukin-4 receptor. Science. 1993;262:1880–1883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

