Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K(+) channels that underlie the neuronal M current
- PMID: 10684873
- PMCID: PMC6772928
- DOI: 10.1523/JNEUROSCI.20-05-01710.2000
Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K(+) channels that underlie the neuronal M current
Abstract
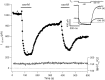
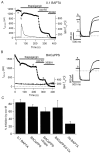
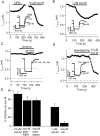
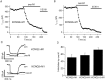
Channels from KCNQ2 and KCNQ3 genes have been suggested to underlie the neuronal M-type K(+) current. The M current is modulated by muscarinic agonists via G-proteins and an unidentified diffusible cytoplasmic messenger. Using whole-cell clamp, we studied tsA-201 cells in which cloned KCNQ2/KCNQ3 channels were coexpressed with M(1) muscarinic receptors. Heteromeric KCNQ2/KCNQ3 currents were modulated by the muscarinic agonist oxotremorine-M (oxo-M) in a manner having all of the characteristics of modulation of native M current in sympathetic neurons. Oxo-M also produced obvious intracellular Ca(2+) transients, observed by using indo-1 fluorescence. However, modulation of the current remained strong even when Ca(2+) signals were abolished by the combined use of strong intracellular Ca(2+) buffers, an inhibitor of IP(3) receptors, and thapsigargin to deplete Ca(2+) stores. Muscarinic modulation was not blocked by staurosporine, a broad-spectrum protein kinase inhibitor, arguing against involvement of protein kinases. The modulation was not associated with a shift in the voltage dependence of channel activation. Homomeric KCNQ2 and KCNQ3 channels also expressed well and were modulated individually by oxo-M, suggesting that the motifs for modulation are present on both channel subtypes. Homomeric KCNQ2 and KCNQ3 currents were blocked, respectively, at very low and at high concentrations of tetraethylammonium ion. Finally, when KCNQ2 subunits were overexpressed by intranuclear DNA injection in sympathetic neurons, total M current was fully modulated by the endogenous neuronal muscarinic signaling mechanism. Our data further rule out Ca(2+) as the diffusible messenger. The reconstitution of muscarinic modulation of the M current that uses cloned components should facilitate the elucidation of the muscarinic signaling mechanism.
Figures




References
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
-
- Bernheim L, Beech DJ, Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991;6:859–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
