Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish
- PMID: 10684889
- PMCID: PMC6772933
- DOI: 10.1523/JNEUROSCI.20-05-01883.2000
Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish
Abstract
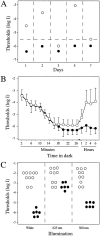
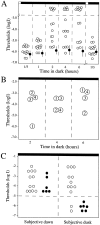
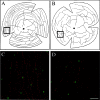
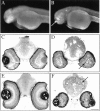
We describe here a dominant mutation, night blindness b (nbb), which causes an age-related visual system defect in zebrafish. At 4-5 months of age, dark-adapted nbb(+/-) mutants show abnormal visual threshold fluctuations when measured behaviorally. Light sensitizes the animals; thus early dark adaptation of nbb(+/-) fish is normal. After 2 hr of dark adaptation, however, visual thresholds of nbb(+/-) mutants are raised on average 2-3 log units, and rod system function is not detectable. Electroretinograms recorded from nbb(+/-) mutants are normal, but ganglion cell thresholds are raised in prolonged darkness, suggesting an inner retinal defect. The visual defect of nbb(+/-) mutants may be likely caused by an abnormal olfactoretinal centrifugal innervation; in nbb(+/-) mutants, the olfactoretinal centrifugal projection to the retina is disrupted, and the number of retinal dopaminergic interplexiform cells is reduced. A similar visual defect as shown by nbb(+/-) mutants is observed in zebrafish in which the olfactory epithelium and olfactory bulb have been excised. Homozygous nbb fish display an early onset neural degeneration throughout the CNS and die by 7-8 d of age.
Figures




References
-
- Arey LB. The function of the afferent fibers of the optic nerve of fishes. J Comp Neurol. 1916;26:213–245.
-
- Baier H, Klostermann S, Trowe T, Karlstrom RO, Nusslein-Volhard C, Bonhoeffer F. Genetic dissection of the retinotectal projection. Development. 1996;123:415–425. - PubMed
-
- Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Branchydanio rerio. I. Structure. J Comp Neurol. 1984;224:107–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
