Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons
- PMID: 10684891
- PMCID: PMC6772909
- DOI: 10.1523/JNEUROSCI.20-05-01904.2000
Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons
Abstract
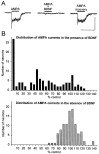
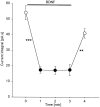
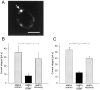
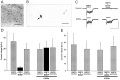
Brain-derived neurotrophic factor (BDNF) is expressed by many primary sensory neurons that no longer require neurotrophins for survival, indicating that BDNF may be used as a signaling molecule by the afferents themselves. Because many primary afferents also express glutamate, we investigated the possibility that BDNF modulates glutamatergic AMPA responses of newborn second-order sensory relay neurons. Perforated-patch, voltage-clamp recordings were made from dissociated neurons of the brainstem nucleus tractus solitarius (nTS), a region that receives massive primary afferent input from BDNF-containing neurons in the nodose and petrosal cranial sensory ganglia. Electrophysiological analysis was combined in some experiments with anterograde labeling of primary afferent terminals to specifically analyze responses of identified second-order neurons. Our data demonstrate that BDNF strongly inhibits AMPA-mediated currents in a large subset of nTS cells. Specifically, AMPA responses were either completely abolished or markedly inhibited by BDNF in 73% of postnatal day (P0) cells and in 82% of identified P5 second-order sensory relay neurons. This effect of BDNF is mimicked by NT-4, but not NGF, and blocked by the Trk tyrosine kinase inhibitor K252a, consistent with a requirement for TrkB receptor activation. Moreover, analysis of TrkB expression in culture revealed a close correlation between the percentage of nTS neurons in which BDNF inhibits AMPA currents and the percentage of neurons that exhibit TrkB immunoreactivity. These data document a previously undefined mechanism of acute modulation of AMPA responses by BDNF and indicate that BDNF may regulate glutamatergic transmission at primary afferent synapses.
Figures
References
-
- Ambalavanar R, Ludlow CL, Wenthold RJ, Tanaka Y, Damirjian M, Petralia RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp Neurol. 1998;402:75–92. - PubMed
-
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. - PubMed
-
- Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7:134–142. - PubMed
-
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
