Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity
- PMID: 10684896
- PMCID: PMC6772942
- DOI: 10.1523/JNEUROSCI.20-05-01952.2000
Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity
Abstract
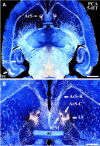
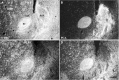
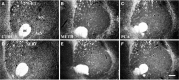
Dopamine release in the nucleus accumbens (NAc) has been implicated as mediating the rewarding effects of stimulant drugs; however, recent studies suggest that 5-HT release may also contribute. In an effort to assess the role of 5-HT in drug-mediated reward, this study analyzed the serotonergic innervation of NAc using immunocytochemistry for 5-HT and the 5-HT transporter (SERT). We report that in control rats the NAc receives two distinct types of 5-HT axons that differ in regional distribution, morphology, and SERT expression. Most regions of the NAc are innervated by thin 5-HT axons that express SERT, but in the caudal NAc shell nearly all 5-HT axons lack SERT and have large spherical varicosities. Two weeks after methamphetamine or p-chloroamphetamine (PCA) treatment, most 5-HT axons in dorsal striatum and NAc have degenerated; however, the varicose axons in the shell appear intact. These drug-resistant 5-HT axons that lack SERT densely innervate the caudal one-third of the accumbens shell, the same location where dopamine axons are spared after methamphetamine. Moreover, 4 hr after PCA, the varicose axons in the caudal shell retain prominent stores of 5-HT, whereas 5-HT axons in the rest of the NAc are depleted of neurotransmitter. The results demonstrate that two functionally different 5-HT projections innervate separate regions of the NAc and that selective vulnerability to amphetamines may result from differential expression of SERT. We postulate that action potentials conducted from the raphe nuclei can release 5-HT throughout the NAc, whereas transporter-mediated release induced by stimulant drugs is more restricted and unlikely to occur in the caudal NAc shell.
Figures





References
-
- Axt KJ, Molliver ME. Immunocytochemical evidence for methamphetamine-induced serotonergic axon loss in the rat brain. Synapse. 1991;9:302–313. - PubMed
-
- Axt KJ, Molliver ME, Qian Y, Blakely RD. Subtypes of 5-HT axons differ in their expression of serotonin transporter. Soc Neurosci Abstr. 1995;21:865.
-
- Benloucif S, Galloway MP. Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur J Pharmacol. 1991;200:1–8. - PubMed
-
- Berridge CW, Stratford TL, Foote SL, Kelley AE. Distribution of dopamine-β-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse. 1997;27:230–241. - PubMed
-
- Blue ME, Yagaloff KA, Mamounas LA, Hartig PR, Molliver ME. Correspondence between 5-HT2 receptors and serotonergic axons in rat neocortex. Brain Res. 1988;453:315–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
