A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia
- PMID: 10684900
- PMCID: PMC6772902
- DOI: 10.1523/JNEUROSCI.20-05-01990.2000
A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia
Abstract
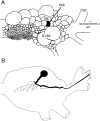
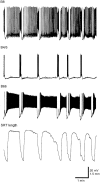
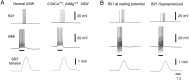
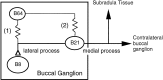
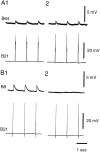
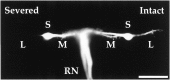
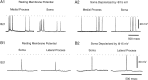
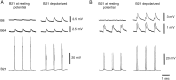
Afferent regulation of centrally generated activity is likely to be more complex than has been established. We show that a neuron that is an exteroceptor can also function as a proprioceptor. We study the Aplysia neuron B21. Previous data suggest that B21 functions as an exteroceptor during the radula closing/retraction phase of ingestive feeding. We show that the tissue innervated by B21, the subradula tissue (SRT), is innervated by a motor neuron (B66) and that B66-induced SRT contractions trigger centripetal spikes in B21. Thus, B21 is also a proprioceptor. To determine whether exteroceptive and proprioceptive activities occur during the same phase of ingestive feeding, we further characterize B66. We show that B66 stimulation does not close or retract the radula. Instead it opens it. Moreover, B66 is electrically coupled to other opening/protraction neurons. Finally, we elicit motor programs in semi-intact preparations and show that during radula opening/protraction we observe B66 activity, SRT contractions, and spikes in B21 that can be eliminated if B66 is indirectly hyperpolarized. B21 is, therefore, likely to act as an exteroceptor during one phase of ingestive feeding and as a proprioceptor during the antagonistic phase. Previous experiments have shown that centripetal spikes in B21 are only transmitted to one follower if they are "gated in" by depolarization. During ingestive programs B21 is centrally depolarized during closing/retraction, but it is not depolarized during opening/protraction. We sought to determine whether there are other followers that receive B21 input when it is not centrally depolarized. We found one such cell. Moreover, we found that stimulation of B21 during radula opening/protraction significantly decreases the duration of this phase of behavior. Thus, proprioceptive activity in B21 is likely to have an impact on motor programs.
Figures









References
-
- Alexeeva A, Borovikov D, Miller MW, Rosen SC, Cropper EC. Effect of a serotonergic extrinsic modulatory neuron (MCC) on radula mechanoafferent function in Aplysia. J Neurophysiol. 1998;80:1609–1622. - PubMed
-
- Baxter DA, Patel VC, Susswein AJ, Bryne J. Computational model of a multifunctional central pattern generator (CPG) that underlies consummatory feeding behavior in Aplysia. Soc Neurosci Abstr. 1997;23:1044.
-
- Borovikov D, Rosen SC, Cropper EC. SCP-containing sensory neurons in Aplysia are peripherally activated as a result of motor neuron activity. Soc Neurosci Abstr. 1997;23:1045.
-
- Busches A, El Manira A. Sensory pathways and their modulation in the control of locomotion. Curr Opin Neurobiol. 1998;18:733–739. - PubMed
-
- Cattaert D, Clarac F. Presynaptic inhibition in crayfish primary afferents. In: Rudomin P, Romo R, Mendell LM, editors. Presynaptic inhibition and neural control. Oxford UP; New York: 1998. pp. 192–205.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
