Assembly of archaeal signal recognition particle from recombinant components
- PMID: 10684931
- PMCID: PMC111052
- DOI: 10.1093/nar/28.6.1365
Assembly of archaeal signal recognition particle from recombinant components
Abstract
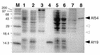
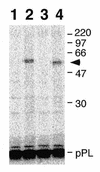
Signal recognition particle (SRP) takes part in protein targeting and secretion in all organisms. Searches for components of archaeal SRP in primary databases and completed genomes indicated that archaea possess only homologs of SRP RNA, and proteins SRP19 and SRP54. A recombinant SRP was assembled from cloned, expressed and purified components of the hyperthermophilic archaeon Archaeoglobus fulgidus. Recombinant Af-SRP54 associated with the signal peptide of bovine pre-prolactin translated in vitro. As in mammalian SRP, Af-SRP54 binding to Af-SRP RNA required protein Af-SRP19, although notable amounts bound in absence of Af-SRP19. Archaeoglobus fulgidus SRP proteins also bound to full-length SRP RNA of the archaeon Methanococcus jannaschii, to eukaryotic human SRP RNA, and to truncated versions which corresponded to the large domain of SRP. Dependence on SRP19 was most pronounced with components from the same species. Reconstitutions with heterologous components revealed a significant potential of human SRP proteins to bind to archaeal SRP RNAs. Surprisingly, M.jannaschii SRP RNA bound to human SRP54M quantitatively in the absence of SRP19. This is the first report of reconstitution of an archaeal SRP from recombinantly expressed purified components. The results highlight structural and functional conservation of SRP assembly between archaea and eucarya.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

