Different roles for abf1p and a T-rich promoter element in nucleosome organization of the yeast RPS28A gene
- PMID: 10684934
- PMCID: PMC111049
- DOI: 10.1093/nar/28.6.1390
Different roles for abf1p and a T-rich promoter element in nucleosome organization of the yeast RPS28A gene
Abstract
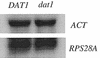
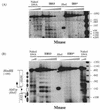
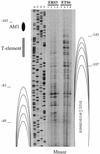
In vivo mutational analysis of the yeast RPS28A ribosomal protein (rp-)gene promoter demonstrated that both the Abf1p binding site and the adjacent T-rich element are essential for efficient transcription. In vivo Mnase and DNaseI digestion showed that the RPS28A promoter contains a 50-60 bp long nucleosome-free region directly downstream from the Abf1p binding site, followed by an ordered array of nucleosomes. Mutating either the Abf1p binding site or the T-rich element has dramatic, but different, effects on the local chromatin structure. Failure to bind Abf1p appears to cause nucleosome positioning to become disorganized as concluded from the complete disappearance of Mnase hypersensitive sites. On the other hand, mutation of the T-rich element causes the downstream nucleosomal array to shift by approximately 50 bp towards the Abf1p site, resulting in loss of the nucleosome-free region downstream of Abf1p. We conclude that Abf1p is a strong organizer of local chromatin structure that appears to act as a nucleosomal boundary factor requiring the downstream T-rich element to create a nucleosome-free region.
Figures


References
-
- Planta R.J., Gonçalves,P.M. and Mager,W.H. (1995) Biochem. Cell Biol., 73, 825–834. - PubMed
-
- Gonçalves P.M., Maurer,K., van Nieuw Amerongen,G., Bergkamp-Steffens,K., Mager,W.H. and Planta,R.J. (1996) Mol. Microbiol., 19, 535–543. - PubMed
-
- Kraakman L.S., Mager,W.H., Grootjans,J.J. and Planta,R.J. (1991) Biochim. Biophys. Acta, 1090, 204–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

