End-to-end diffusion on the microsecond timescale measured with resonance energy transfer from a long-lifetime rhenium metal-ligand complex
- PMID: 10687388
- PMCID: PMC6816249
- DOI: 10.1562/0031-8655(2000)071<0157:etedot>2.0.co;2
End-to-end diffusion on the microsecond timescale measured with resonance energy transfer from a long-lifetime rhenium metal-ligand complex
Abstract
We measured the end-to-end diffusion coefficient of an alkyl chain-linked donor-acceptor pair using the time-resolved frequency-domain decay of the donor. The donor was a rhenium metal-ligand complex with a mean decay time ranging from 2.1 to 7.9 microseconds in the absence of the Texas red acceptor. The decay time was used to measure the donor-to-acceptor distance distribution and the mutual diffusion coefficient. Using this long lifetime donor, it was easily possible to determine a diffusion coefficient near 2 x 10(-8) cm2/s and diffusion coefficients as low as 1.3 x 10(-9) cm2/s were measurable. Such long lifetime donors should be valuable for measuring the flexing of peptides on the microsecond timescale, domain motions of proteins and lateral diffusion in membranes. The availability of microsecond decay time luminophores now allows luminescence spectroscopy to be useful generally for studies of microsecond dynamics of biological macromolecules.
Figures




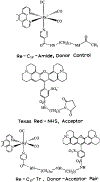
References
-
- Demchenko AP (1994) Protein fluorescence, dynamics and function: exploration of analogy between electronically excited and biocatalytic transition states. Biochim. Biophys. Acta 1209, 149–164. - PubMed
-
- McCammon JA and Harvey SC (1987) Dynamics of Proteins and Nucleic Acids. Cambridge University Press, New York.
-
- Hochstrasser RM (1998) Ultrafast spectroscopy of protein dynamics. J. Chem. Ed 75, 559–564.
-
- Demchenko AP (1992) Fluorescence and dynamics in pro teins In Topics in Fluorescence Spectroscopy, Vol. 3: Biochemical Applications (Edited by Lakowicz JR), pp. 65–111. Plenum Press, New York.
-
- Stubbs CD and Williams BW (1992) Fluorescence in membranes In Topics in Fluorescence Spectroscopy, Vol. 3: Biochemical Applications (Edited by Lakowicz JR), pp. 231–271. Plenum Press, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

