An AU-rich sequence element (UUUN[A/U]U) downstream of the edited C in apolipoprotein B mRNA is a high-affinity binding site for Apobec-1: binding of Apobec-1 to this motif in the 3' untranslated region of c-myc increases mRNA stability
- PMID: 10688645
- PMCID: PMC110815
- DOI: 10.1128/MCB.20.6.1982-1992.2000
An AU-rich sequence element (UUUN[A/U]U) downstream of the edited C in apolipoprotein B mRNA is a high-affinity binding site for Apobec-1: binding of Apobec-1 to this motif in the 3' untranslated region of c-myc increases mRNA stability
Abstract
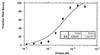
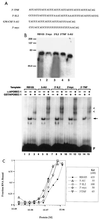
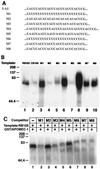
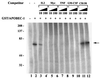
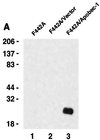
Apobec-1, the catalytic subunit of the mammalian apolipoprotein B (apoB) mRNA-editing enzyme, is a cytidine deaminase with RNA binding activity for AU-rich sequences. This RNA binding activity is required for Apobec-1 to mediate C-to-U RNA editing. Filter binding assays, using immobilized Apobec-1, demonstrate saturable binding to a 105-nt apoB RNA with a K(d) of approximately 435 nM. A series of AU-rich templates was used to identify a high-affinity ( approximately 50 nM) binding site of consensus sequence UUUN[A/U]U, with multiple copies of this sequence constituting the high-affinity binding site. In order to determine whether this consensus site could be functionally demonstrated from within an apoB RNA, circular-permutation analysis was performed, revealing one major (UUUGAU) and one minor (UU) site located 3 and 16 nucleotides, respectively, downstream of the edited base. Secondary-structure predictions reveal a stem-loop flanking the edited base with Apobec-1 binding to the consensus site(s) at an open loop. A similar consensus (AUUUA) is present in the 3' untranslated regions of several mRNAs, including that of c-myc, that are known to undergo rapid degradation. In this context, it is presumed that the consensus motif acts as a destabilizing element. As an independent test of the ability of Apobec-1 to bind to this sequence, F442A cells were transfected with Apobec-1 and the half-life of c-myc mRNA was determined following actinomycin D treatment. These studies demonstrated an increase in the half-life of c-myc mRNA from 90 to 240 min in control versus Apobec-1-expressing cells. Apobec-1 expression mutants, in which RNA binding activity is eliminated, failed to alter c-myc mRNA turnover. Taken together, the data establish a consensus binding site for Apobec-1 embedded in proximity to the edited base in apoB RNA. Binding to this site in other target RNAs raises the possibility that Apobec-1 may be involved in other aspects of RNA metabolism, independent of its role as an apoB RNA-specific cytidine deaminase.
Figures






References
-
- Anant S, MacGinnitie A J, Davidson N O. apobec-1, the catalytic subunit of the mammalian apolipoprotein B mRNA editing enzyme, is a novel RNA-binding protein. J Biol Chem. 1995;270:14762–14767. - PubMed
-
- Anant S, MacGinnitie A J, Davidson N O. The binding of apobec-1 to mammalian apo B RNA is stabilized by the presence of complementation factors which are required for posttranscriptional editing. Nucleic Acids Symp Ser. 1995;33:99–102. - PubMed
-
- Backus J W, Schock D, Smith H C. Only cytidines 5′ of the apolipoprotein B mRNA mooring sequence are edited. Biochim Biophys Acta. 1994;1219:1–14. - PubMed
-
- Bhattacharya S, Navaratnam N, Morrison J R, Scott J, Taylor W R. Cytosine nucleoside/nucleotide deaminases and apolipoprotein B mRNA editing. Trends Biochem Sci. 1994;19:105–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
