Two independent signaling pathways mediate the antiapoptotic action of macrophage-stimulating protein on epithelial cells
- PMID: 10688668
- PMCID: PMC110838
- DOI: 10.1128/MCB.20.6.2218-2227.2000
Two independent signaling pathways mediate the antiapoptotic action of macrophage-stimulating protein on epithelial cells
Abstract
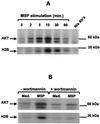
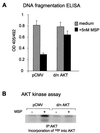
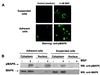
In addition to its effects on macrophage function, macrophage-stimulating protein (MSP) is a growth and motility factor for epithelial cells. The growth and survival of epithelial cells generally require two signals, one generated by interaction with extracellular matrix via integrins, the other initiated by a growth factor. Therefore we investigated the effect of MSP on epithelial cell survival. Survival of epithelial cells cultured overnight in serum-free medium was promoted by adhesion, which activated both the phosphatidylinositol 3'-kinase (PI3-K)/AKT and mitogen-activated protein kinase (MAPK) pathways, operating independently of one another. The number of apoptotic cells resulting from inhibition of either pathway alone was approximately doubled by simultaneous inhibition of both pathways. This shows that each pathway made a partial contribution to the prevention of apoptosis. In the presence of an inhibitor of either pathway, MSP increased the activity of the other pathway so that the single uninhibited pathway alone was sufficient to prevent apoptosis. In contrast to the results with adherent cells, although MSP also prevented apoptosis of cells in suspension (anoikis), its effect was mediated only by the PI3-K/AKT pathway. Despite activation of MAPK by MSP, anoikis was not prevented in suspended cells with a blocked PI3-K/AKT pathway. Thus, activation of MAPK alone is not sufficient to mediate MSP antiapoptotic effects. Cell adhesion generates an additional signal, which is essential for MSP to use MAPK in an antiapoptotic pathway. This may involve translocation of MSP-activated MAPK from the cytoplasm into the nucleus, which occurs only in adherent cells. Our results suggest that there is cross talk between cell matrix adhesion and growth factors in the regulation of cell survival via the MAPK pathway. Growth factors induce MAPK activation, and adhesion mediates MAPK translocation from the cytoplasm into the nucleus.
Figures




References
-
- Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. - PubMed
-
- Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Aplin A E, Juliano R L. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J Cell Sci. 1999;112:695–706. - PubMed
-
- Assoian R K, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol. 1997;9:93–98. - PubMed
-
- Berra E, Diaz-Meco M T, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. . (Erratum, 273:16630.) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
