Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter
- PMID: 10688670
- PMCID: PMC110840
- DOI: 10.1128/MCB.20.6.2239-2247.2000
Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter
Abstract
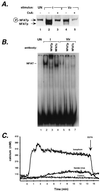
The human tumor necrosis factor alpha (TNF-alpha) gene is rapidly activated in response to multiple signals of stress and inflammation. We have identified transcription factors present in the TNF-alpha enhancer complex in vivo following ionophore stimulation (ATF-2/Jun and NFAT) and virus infection (ATF-2/Jun, NFAT, and Sp1), demonstrating a novel role for NFAT and Sp1 in virus induction of gene expression. We show that virus infection results in calcium flux and calcineurin-dependent NFAT dephosphorylation; however, relatively lower levels of NFAT are present in the nucleus following virus infection as compared to ionophore stimulation. Strikingly, Sp1 functionally synergizes with NFAT and ATF-2/c-jun in the activation of TNF-alpha gene transcription and selectively associates with the TNF-alpha promoter upon virus infection but not upon ionophore stimulation in vivo. We conclude that the specificity of TNF-alpha transcriptional activation is achieved through the assembly of stimulus-specific enhancer complexes and through synergistic interactions among the distinct activators within these enhancer complexes.
Figures






References
-
- Aderka D, Holtmann H, Toker L, Hahn T, Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986;136:2938–2942. - PubMed
-
- Aggarwal B B, Puri R K. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Science; 1995.
-
- Beals C R, Clipstone N A, Ho S N, Crabtree G R. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 1997;11:824–834. - PubMed
-
- Brinkman B M N, Telliez J-B, Schievella A R, Lin L-L, Goldfeld A E. Engagement of TNF receptor 1 leads to ATF-2 and p38 MAP kinase-dependent TNF-α gene expression. J Biol Chem. 1999;274:30882–30886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
