The amino terminus of the mixed lineage leukemia protein (MLL) promotes cell cycle arrest and monocytic differentiation
- PMID: 10688900
- PMCID: PMC16009
- DOI: 10.1073/pnas.040574897
The amino terminus of the mixed lineage leukemia protein (MLL) promotes cell cycle arrest and monocytic differentiation
Abstract
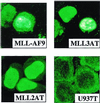
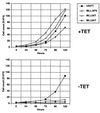
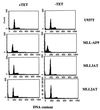
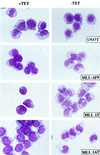
Several lines of evidence suggest that the mixed lineage leukemia protein (MLL, ALL-1, HRX) plays a role in regulating myelomonocytic differentiation. In this study we examined the effect of expression of MLL-AF9 on differentiation of the monoblastic U937 cell line by using a tetracycline-inducible expression system. MLL-AF9 arrested growth of U937 cells and induced these cells to differentiate into macrophages; induction was accompanied by expression of CD11b and CD14 and ultimately cell death. Deletion mutants of MLL-AF9 were used to map the sequences responsible for this effect. The amino-terminal half of MLL was sufficient for both cell cycle arrest and macrophage differentiation, whereas the carboxyl terminus of MLL or AF9 was found to be dispensable for this effect. Further deletions showed that a 35-kDa amino-terminal fragment spanning two AT hook motifs was sufficient for cell cycle arrest, up-regulation of p21(Cip1) and p27(Kip1), and partial differentiation toward macrophages. These findings suggest a possible role for the MLL AT hook-containing region in regulating myelomonocytic differentiation.
Figures




References
-
- Waring P M, Cleary M L. Curr Topics Microbiol Immunol. 1997;220:1–23. - PubMed
-
- Bernard O A, Berger R. Genes Chromosomes Cancer. 1995;13:75–85. - PubMed
-
- Felix C A, Winick N J, Negrini M, Bowman W P, Croce C M, Lange B J. Cancer Res. 1993;53:2954–2956. - PubMed
-
- Gill-Super H J, McCabe N R, Thirman M J, Larson R A, LeBeau M M, Pedersen-Bjergaard J, Philip P, Diaz M O, Rowley J D. Blood. 1993;82:3705–3711. - PubMed
-
- Djabali M, Selleri L, Parry P, Bower M, Young B D, Evans G A. Nat Genet. 1992;2:113–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

