Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers
- PMID: 10688907
- PMCID: PMC16006
- DOI: 10.1073/pnas.050569097
Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers
Abstract
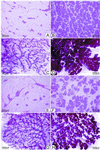
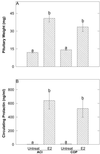
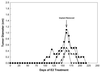
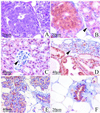
The genetically related ACI and Copenhagen (COP) rat strains display diametrically opposed susceptibilities to mammary cancer development when treated chronically with 17beta-estradiol (E2). Here, we compare the actions of E2 on cell proliferation and lobuloalveolar development in the mammary glands of female ACI and COP rats. After 12 wk of E2 treatment, the mammary glands of ACI rats exhibited a significantly greater proliferative response to E2, compared with COP rats, as evidenced by quantification of S phase fraction and development of lobuloalveolar hyperplasia. Focal regions of atypical epithelial hyperplasia were observed in ACI, but not COP, rats. These strain differences were not because of differences in circulating E2, progesterone or, prolactin. Two-thirds of the induced mammary cancers in ACI rats exhibited aneuploidy. The E2-induced mammary cancers regressed when hormone treatment was discontinued, indicating that they were estrogen-dependent. Progesterone receptor was expressed by the great majority of epithelial cells within the E2-induced atypical hyperplastic foci and the mammary carcinomas, suggesting a link between these lesions. These data demonstrate a correlation between E2 action in the induction of mammary cell proliferation and atypical epithelial hyperplasia and genetically conferred susceptibility to E2-induced mammary cancers.
Figures


References
-
- Harris J R, Lippman M E, Veronesi U, Willet W. N Engl J Med. 1992;327:319–328. - PubMed
-
- Pike M C, Spicer D V, Dahmoush L, Press M F. Epidemiol Rev. 1993;15:17–35. - PubMed
-
- Bernstein L, Ross R K. Epidemiol Rev. 1993;15:48–65. - PubMed
-
- Fisher B, Costantino J P, Wickerham D L, Redmond C K, Kavanah M, Cronin W M, Vogel V, Robidoux A, Dimitrov N, Atkins J, et al. J Natl Cancer Inst. 1998;90:1371–1388. - PubMed
-
- Dunning W F, Curtis M R, Segaloff A. Cancer Res. 1947;7:511–521.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

