The carboxyl terminus of vertebrate poly(A) polymerase interacts with U2AF 65 to couple 3'-end processing and splicing
- PMID: 10691733
- PMCID: PMC316384
The carboxyl terminus of vertebrate poly(A) polymerase interacts with U2AF 65 to couple 3'-end processing and splicing
Abstract
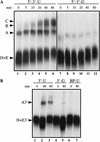
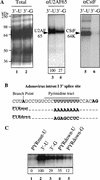
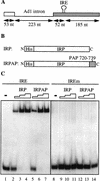
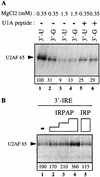
Although it has been established that the processing factors involved in pre-mRNA splicing and 3'-end formation can influence each other positively, the molecular basis of this coupling interaction was not known. Stimulation of pre-mRNA splicing by an adjacent cis-linked cleavage and polyadenylation site in HeLa cell nuclear extract is shown to occur at an early step in splicing, the binding of U2AF 65 to the pyrimidine tract of the intron 3' splice site. The carboxyl terminus of poly(A) polymerase (PAP) previously has been implicated indirectly in the coupling process. We demonstrate that a fusion protein containing the 20 carboxy-terminal amino acids of PAP, when tethered downstream of an intron, increases splicing efficiency and, like the entire 3'-end formation machinery, stimulates U2AF 65 binding to the intron. The carboxy-terminal domain of PAP makes a direct and specific interaction with residues 17-47 of U2AF 65, implicating this interaction in the coupling of splicing and 3'-end formation.
Figures


References
-
- Abovich N, Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. - PubMed
-
- Arias JA, Peterson SR, Dynan WS. Promoter-dependent phosphorylation of RNA polymerase II by a template-bound kinase. Association with transcriptional initiation. J Biol Chem. 1991;266:8055–8061. - PubMed
-
- Barabino SML, Blencowe BJ, Ryder U, Sproat BS, Lamond AI. Targeted snRNP depletion reveals an additional role for mammalian U1 snRNP in spliceosome assembly. Cell. 1990;63:293–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
