Trypanosoma cruzi-induced immunosuppression: B cells undergo spontaneous apoptosis and lipopolysaccharide (LPS) arrests their proliferation during acute infection
- PMID: 10691924
- PMCID: PMC1905583
- DOI: 10.1046/j.1365-2249.2000.01150.x
Trypanosoma cruzi-induced immunosuppression: B cells undergo spontaneous apoptosis and lipopolysaccharide (LPS) arrests their proliferation during acute infection
Abstract
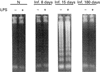
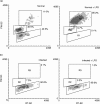
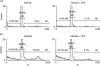
Acute infection with Trypanosoma cruzi is characterized by multiple manifestations of immunosuppression of both cellular and humoral responses. B cells isolated at the acute stage of infection have shown marked impairment in their response to polyclonal activators in vitro. The present work aims at studying the B cell compartment in the context of acute T. cruzi infection to provide evidence for B cell activation, spontaneous apoptosis and arrest of the cell cycle upon mitogenic stimulation as a mechanism underlying B cell hyporesponse. We found that B cells from acutely infected mice, which fail to respond to the mitogen LPS, showed spontaneous proliferation and production of IgM, indicating a high level of B cell activation. Furthermore, these activated B cells also exhibited an increase in Fas expression and apoptosis in cultures without an exogenous stimulus. On the other hand, B cells from early acute and chronic infected mice did not present activation or apoptosis, and were able to respond properly to the mitogen. Upon in vitro stimulation with LPS, B cells from hyporesponder mice failed to progress through the cell cycle (G0/G1 arrest), nor did they increase the levels of apoptosis. These results indicate that B cell apoptosis and cell cycle arrest could be the mechanisms that control intense B cell expansion, but at the same time could be delaying the emergence of a specific immune response against the parasite.
Figures
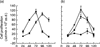



Similar articles
-
Specific humoral immunity versus polyclonal B cell activation in Trypanosoma cruzi infection of susceptible and resistant mice.PLoS Negl Trop Dis. 2010 Jul 6;4(7):e733. doi: 10.1371/journal.pntd.0000733. PLoS Negl Trop Dis. 2010. PMID: 20625554 Free PMC article.
-
A Trypanosoma cruzi membrane protein shares an epitope with a lymphocyte activation antigen and induces crossreactive antibodies.J Exp Med. 1992 Jun 1;175(6):1473-82. doi: 10.1084/jem.175.6.1473. J Exp Med. 1992. PMID: 1375261 Free PMC article.
-
Trypanosoma cruzi infection selectively renders parasite-specific IgG+ B lymphocytes susceptible to Fas/Fas ligand-mediated fratricide.J Immunol. 2002 Apr 15;168(8):3965-73. doi: 10.4049/jimmunol.168.8.3965. J Immunol. 2002. PMID: 11937553
-
Trypanosoma cruzi infection beats the B-cell compartment favouring parasite establishment: can we strike first?Scand J Immunol. 2007 Aug-Sep;66(2-3):137-42. doi: 10.1111/j.1365-3083.2007.01968.x. Scand J Immunol. 2007. PMID: 17635791 Review.
-
Apoptosis as a cause of T-cell unresponsiveness in experimental Chagas' disease.Braz J Med Biol Res. 1995 Aug;28(8):913-8. Braz J Med Biol Res. 1995. PMID: 8555995 Review.
Cited by
-
Regulated expression and effect of galectin-1 on Trypanosoma cruzi-infected macrophages: modulation of microbicidal activity and survival.Infect Immun. 2001 Nov;69(11):6804-12. doi: 10.1128/IAI.69.11.6804-6812.2001. Infect Immun. 2001. PMID: 11598053 Free PMC article.
-
Immunophenotypic lymphocyte profiles in human african trypanosomiasis.PLoS One. 2009 Jul 8;4(7):e6184. doi: 10.1371/journal.pone.0006184. PLoS One. 2009. PMID: 19584913 Free PMC article.
-
Fbxl12 triggers G1 arrest by mediating degradation of calmodulin kinase I.Cell Signal. 2013 Oct;25(10):2047-59. doi: 10.1016/j.cellsig.2013.05.012. Epub 2013 May 23. Cell Signal. 2013. PMID: 23707388 Free PMC article.
-
Glycoinositolphospholipids from Trypanosoma cruzi induce B cell hyper-responsiveness in vivo.Glycoconj J. 2000 Oct;17(10):727-34. doi: 10.1023/a:1011082925179. Glycoconj J. 2000. PMID: 11425193
-
3-Hydroxy kynurenine treatment controls T. cruzi replication and the inflammatory pathology preventing the clinical symptoms of chronic Chagas disease.PLoS One. 2011;6(10):e26550. doi: 10.1371/journal.pone.0026550. Epub 2011 Oct 19. PLoS One. 2011. PMID: 22028903 Free PMC article.
References
-
- Ramos CE, Lamoy M, Feoly M, Rodriguez M, Perez M, Ortiz-Ortiz L. T. cruzi: immunosuppressed response to different antigens in the infected mouse. Exp Parasitol. 1978;45:190–7. - PubMed
-
- Minoprio P, Itohara S, Heusser C, Tonegawa S, Countinho A. Immunobiology of murine T. cruzi infection: the predominance of parasite nonspecific responses and the activation of TcR I T cells. Immunol Rev. 1989;112:184–206. - PubMed
-
- Krettli AU, Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infection. J Immunol. 1976;116:755–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

