Molecular basis for the temperature sensitivity of Escherichia coli pth(Ts)
- PMID: 10692356
- PMCID: PMC94448
- DOI: 10.1128/JB.182.6.1523-1528.2000
Molecular basis for the temperature sensitivity of Escherichia coli pth(Ts)
Abstract
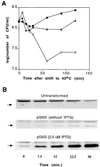
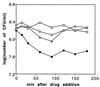
The gene pth, encoding peptidyl-tRNA hydrolase (Pth), is essential for protein synthesis and viability of Escherichia coli. Two pth mutants have been studied in depth: a pth(Ts) mutant isolated as temperature sensitive and a pth(rap) mutant selected as nonpermissive for bacteriophage lambda vegetative growth. Here we show that each mutant protein is defective in a different way. The Pth(Ts) protein was very unstable in vivo, both at 43 degrees C and at permissive temperatures, but its specific activity was comparable to that of the wild-type enzyme, Pth(wt). Conversely, the mutant Pth(rap) protein had the same stability as Pth(wt), but its specific activity was low. The thermosensitivity of the pth(Ts) mutant, presumably, ensues after Pth(Ts) protein levels are reduced at 43 degrees C. Conditions that increased the cellular Pth(Ts) concentration, a rise in gene copy number or diminished protein degradation, allowed cell growth at a nonpermissive temperature. Antibiotic-mediated inhibition of mRNA and protein synthesis, but not of peptidyl-tRNA drop-off, reduced pth(Ts) cell viability even at a permissive temperature. Based on these results, we suggest that Pth(Ts) protein, being unstable in vivo, supports cell viability only if its concentration is maintained above a threshold that allows general protein synthesis.
Figures

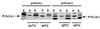
References
-
- Anderson R P, Menninger J R. Test of the ribosome editor hypothesis. III. A mutant Escherichia coli with a defective ribosome editor. Mol Gen Genet. 1987;209:313–318. - PubMed
-
- Armstrong D J, Roman A. Mutagenesis of human papillomavirus types 6 and 16 E7 open reading frames alters the electrophoretic mobility of the expressed proteins. J Gen Virol. 1992;73:1275–1279. - PubMed
-
- Atherly A G, Menninger J R. Mutant E. coli strain with temperature sensitive peptidyl-transfer RNA hydrolase. Nat New Biol. 1972;240:245–246. - PubMed
-
- Chen S M, Takiff H E, Barber A M, Dubois G C, Bardwell J C A, Court D L. Expression and characterization of RNase III and Era proteins. J Biol Chem. 1990;265:2888–2895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

